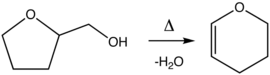- Dihydropyran
-
Dihydropyran 

3,4-dihydropyran
3,6-dihydropyran3,4-Dihydro-2H-pyran
3,6-dihydro-2H-pyranOther names2,3-Dihydro-4H-pyranIdentifiers CAS number 110-87-2 
PubChem 8080, 520540 ChemSpider 7789 
Jmol-3D images Image 1 - O1\C=C/CCC1
- InChI=1/C5H8O/c1-2-4-6-5-3-1/h2,4H,1,3,5H2
Key: BUDQDWGNQVEFAC-UHFFFAOYAJ
Properties Molecular formula C5H8O Molar mass 84.12 Appearance colorless liquid Density 0.992 g/mL at 25 °C Melting point -70 °C, 203 K, -94 °F
Boiling point 86 °C, 359 K, 187 °F
Solubility in water organic solvents  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dihydropyran is a heterocyclic compound with the formula C5H8O. The six-membered, not aromatic, ring has five carbon atoms and one oxygen atom. It contains one double bond. There are two isomers of dihydropyran that differ by the location of the double bond. 3,4-Dihydro-2H-pyran has a double bond at position 5; 3,6-dihydro-2H-pyran has the double bond at position 4.
Contents
Nomenclature
In IUPAC names, "dihydro" refers to the two added hydrogen atoms needed to remove one double bond from the parent compound pyran. The numbers in front of the prefix indicate the position of the added hydrogen atoms. [1] The italicized capital H denotes the "indicated hydrogen", that is a second hydrogen atom present on the location where no double bond is present. The indicated hydrogen is placed just in front of the parent hydride (pyran). [2]
Note that the position numbers of the ring-members not follow the position of the double bonds here. If also the second double bond is removed by two more hydrogen atoms we get tetrahydropyran, or oxane.
3,4-Dihydropyran
3,4-Dihydropyran, also known as 2,3-dihydropyran, is used for protecting several chemicals.[3]
Preparation
Dihydropyran is prepared by the dehydration of tetrahydrofurfuryl alcohol over alumina at 300-400 °C.[4]
Reactions
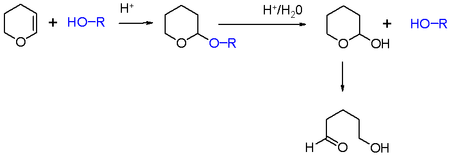
In organic synthesis, the 2-tetrahydropyranyl group is used as a protecting group for alcohols.[5][6] Reaction of the alcohol with dihydropyran forms a tetrahydropyranyl ether, protecting the alcohol from a variety of reactions. The alcohol can later be restored readily by acidic hydrolysis with formation of 5-hydroxypentanal.
See also
References
- ^ A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993): R-3.1.2 Hydro prefixes
- ^ A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993): R-1.3 Indicated Hydrogen
- ^ Technical information from Penn Chemicals
- ^ R. L. Sawyer and D. W. Andrus (1955), "2,3-Dihydropyran", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0276; Coll. Vol. 3: 276
- ^ R. A. Earl L. B. Townsend (1990), "Methyl 4-Hydroxy-2-butynoate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0334; Coll. Vol. 7: 334
- ^ Arthur F. Kluge (1990), "Diethyl [(2-Tetrahydropyranyloxy)methyl]phosphonate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0160; Coll. Vol. 7: 160
Categories:- Dihydropyrans
Wikimedia Foundation. 2010.