- Methane (data page)
-
This page provides supplementary chemical data on methane.Contents
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as SIRI, and follow its directions.
Structure and properties
Structure and properties Index of refraction, nD ? Dielectric constant, εr 1.7 ε0 at ? °C Bond strength ? Bond length .117 nm Bond angle 109.5 Magnetic susceptibility ? Thermodynamic properties
Phase behavior Triple point 90.67 K (−182.48 °C), 0.117 bar Critical point 190.6 K (−82.6 °C), 46 bar Std enthalpy change
of fusion, ΔfusHo1.1 kJ/mol Std entropy change
of fusion, ΔfusSo? J/(mol·K) Std enthalpy change
of vaporization, ΔvapHo8.17 kJ/mol Std entropy change
of vaporization, ΔvapSo? J/(mol·K) Solid properties Std enthalpy change
of formation, ΔfHosolid? kJ/mol Standard molar entropy,
Sosolid? J/(mol K) Heat capacity, cp ? J/(mol K) Liquid properties Std enthalpy change
of formation, ΔfHoliquid? kJ/mol Standard molar entropy,
Soliquid? J/(mol K) Heat capacity, cp ? J/(mol K) Gas properties Std enthalpy change
of formation, ΔfHogas−74.87 kJ/mol Standard molar entropy,
Sogas186.61 J/(mol K) Enthalpy of combustion ΔcH o–882.0 kJ/mol Heat capacity, cp 35.69 J/(mol K) van der Waals' constants[1] a = 228.29 L2 kPa/mol2
b = 0.04278 liter per moleVapor pressure of liquid
P in mm Hg 1 10 40 100 400 760 1520 3800 7600 15200 30400 45600 T in °C –205.9(s) –195.5(s) –187.7(s) –181.4 –168.8 –161.5 –152.3 –138.3 –124.8 –108.5 –86.3 — Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. Annotation "(s)" indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid. Note that these are all negative temperature values.
 log10 of methane vapor pressure vs. temperature. Uses formula:
log10 of methane vapor pressure vs. temperature. Uses formula:
 given in Lange's Handbook of Chemistry, 10th ed. Note that formula loses accuracy near Tcrit = −82.6°C
given in Lange's Handbook of Chemistry, 10th ed. Note that formula loses accuracy near Tcrit = −82.6°CSpectral data
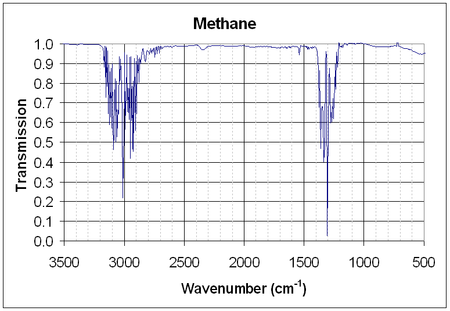
UV-Vis λmax ? nm Extinction coefficient, ε ? IR Major absorption bands ~3020 cm−1 ~1300 NMR Proton NMR Carbon-13 NMR Other NMR data MS Masses of
main fragmentsReferences
- ^ Lange's Handbook of Chemistry, 10th ed, pp 1522-1524
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
Categories:- Chemical data pages
- Methane
Wikimedia Foundation. 2010.