- Laundry detergent
-
Laundry detergent, or washing powder, is a substance that is a type of detergent (cleaning agent) that is added for cleaning laundry. In common usage, "detergent" refers to mixtures of chemical compounds including alkylbenzenesulfonates, which are similar to soap but are less affected by "hard water." In most household contexts, the term detergent refers to laundry detergent vs hand soap or other types of cleaning agents. Most detergent is delivered in powdered form.[1]
Contents
History
From ancient times, chemical additives were recognized for their ability to facilitate the mechanical washing with water. The Italians used a mix of sulfur and water with charcoal to clean cloth. Egyptians added ashes and silicates to soften water. Soaps were the first detergents.[2] The detergent effects of certain synthetic surfactants were noted in Germany in 1917, in response to shortages of soap during World War I. In the 1930s, commercially viable routes to fatty alcohols were developed, and these new materials were converted to their sulfate esters, key ingredients in the commercially important German brand FEWA, produced by BASF, and Dreft, the US brand produced by Procter and Gamble. Such detergents were mainly used in industry until after World War II. By then, new developments and the later conversion of aviation fuel plants to produce tetrapropylene, used in household detergents, caused a fast growth of domestic use in the late 1940s.[3]
The use of enzymes for laundry was introduced in the early part of the 1900s by Otto Rohm. Only in the latter part of the century with the availability of thermally robust bacterial enzymes did this technology become mainstream.[4]
At the present time, soap has largely been displaced as the main cleaning agent in developed countries. Soap is, by weight, relatively ineffective, and it is highly sensitive to deactivation by hard water. By the 1950s, soap had almost been completely replaced by branched alkylbenzenesulfonates, but these detergents were found to be poorly biodegradable. Linear alkylbenzenesulfonates (LABs), however, proved to be both highly effective in cleaning and more biodegradable than the branched relatives. LABs remain the main detergents used domestically. Other detergents that have been developed include the linear alkylsulfonates and olefinsulfonates, which also resist deactivation by hard water. Both remain specialty products, for example only an estimated 60 million kilograms of the sodium alkylsulfonates are produced annually.[5] During the early development of non-soap surfactants as commercial cleaning products, the term syndet, short for synthetic detergent, was promoted to indicate the distinction from so-called natural soaps.
Chemistry of detergents
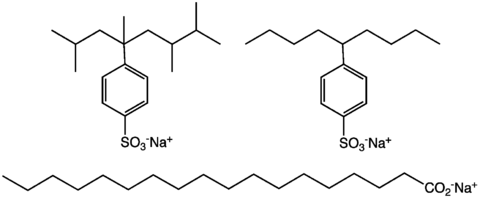
Main article: detergentMany kinds of molecules and ions can serve as high-efficiency surfactants. They are often classified according to the charge of the molecule or ion, the three main classes being anionic, neutral, and cationic detergents. Anionic detergents are most commonly encountered for domestic laundry detergents. Detergents are ions or molecules that contain both polar and nonpolar components. The polar component allows the detergent to dissolve in the water, whereas the nonpolar portion solubilizes greasy ("hydrophobic") materials that are the usual target of the cleaning process. An estimated 6 billion kilograms of detergents are produced annually for domestic markets.[5]
Components
Modern detergent formulations - the entire product vs just the surfactant - contain several components. Three main ingredients are builders (50% by weight, approximately), the alkylbenzenesulfonate surfactant (15%), and bleaches (7%).[5]
Builders
Builders are water softeners. These chemical compounds are agents that remove calcium ions by complexation or precipitation. Typical builders are sodium carbonate, complexation agents, soap, and zeolites. They function by sequestering or precipitating the problematic ions. One of the most common builders is sodium triphosphate, which is used on very large scale for this application.
Bleach
Main article: BleachThe main targets of bleaches are of vegetable origin and include chlorophyll, anthocyanin dyes, tannins, humic acids, and carotenoid pigments. Most bleaches in laundry detergents are oxidizers, e.g., sodium perborate or sodium hypochlorite, In addition, other agents are added as "bleach activators", to enhance the effectiveness of the bleaching agent; a popular one is tetraacetylethylenediamine.
Enzymes
Main article: Biological detergentMany laundry detergents contain enzymes. The amounts of enzyme can be up to about 2% by weight of the product. These agents are required to degrade recalcitrant stains composed of proteins, fats, or carbohydrates. Each type of stain requires a different type of enzyme, i.e., protease for proteins, lipases for greases, and amylases for carbohydrates.
Other ingredients
Many other ingredients are added depending on the specific application. Such additives modify the foaming properties of the product by either stabilizing or counteracting foam. Other ingredients increase or decrease the viscosity of the solution, or solubilize other ingredients. Corrosion inhibitors counteract damage to washing equipment. "Dye transfer inhibitors" prevent dyes from one article from colouring other items. "Antiredeposition agents" are used to prevent fine soil particles from reattaching to the product being cleaned. Carboxymethyl cellulose is used for this purpose.[5]
A number of ingredients affect aesthetic properties of the item to be cleaned or the detergent itself before or during use. These agents include optical brighteners, fabric softeners, and colourants. A variety of perfumes are also components of modern detergents, provided that they are compatible with the other components and do not affect the colour of the cleaned item. The perfumes are typically a mixture of many compounds, a popular component being cyclohexyl salicylate, which is related to oil of wintergreen.[5]
Environmental concerns
Early in the introduction of sulfonate-based detergents, concerns were voiced over the low rates of biodegradation of the branched alkylbenzenesulfonates. This problem was addressed by the introduction of linear alkylbenzenesulfonates.[6]
A more profound problem arises from the heavy use of sodium triphosphate, which can comprise up to 50% by weight of detergents. The discharge of soluble phosphates into natural waters has led to problem with eutrophication of lakes and streams. The replacement of sodium triphosphate by zeolites offers some relief to this problem.[5] With respect to the phosphate additives, between 1940 and 1970 "the amount of phosphates in city wastewater increased from 20,000 to 150,000 tons per year."[7] With the increase in phosphates, algal blooms grew splendidly on the excess phosphorus and consumed most of the oxygen in the waters, killing fish and plants.[7]
In 2004, the European Union introduced regulations to require biodegradability in all detergents,[8] and intends to ban phosphates in domestic products from 2013.[9]
Australia began phasing out the use of phosphates in its detergents in 2011, with an all-out ban expected to take effect in 2014.[10]
Pursuant to findings published in 2006 by the Shenkar College of Engineering and Design indicating that liquid detergents are "much more environment-friendly" than powdered detergents, Israel's Ministry of the Environment began recommending that consumers prefer liquid detergent over powdered ones "for laundry which is not heavily stained."[11]
References
- ^ Handbook of Detergents, Part A, Editor-in-chief: Uri Zoller. Volume editor: Guy Broze, Marcel Dekker, NY: 1999. isbn 0-8247-1417-2
- ^ A variety of agents were used in ancient times, and even (putrescent) urine for certain applications as well as saponins and ox bile. von Georgievics, Georg; Charles Thomas Colley Salter (1902) (Google books), The chemical technology of textile fibres, Scott, Greenwood, p. 81, http://books.google.com/?id=OtxBAAAAIAAJ
- ^ Spriggs, John (July 1975) (pdf), An economical analysis of the developmente of substitutes with some illustrative examples and implications for the beef industry, Staff paper series, University of Minnesota, pp. 34–37, http://ageconsearch.umn.edu/bitstream/123456789/22851/1/p75-14.pdf, retrieved 9 May 2008
- ^ US 3451935, Roald, Arnvid S. & Nicolaas T. DE. Oude, "Granular enzyme-containing laundry composition", issued 24 June 1969
- ^ a b c d e f Eduard Smulders, Wolfgang Rybinski, Eric Sung, Wilfried Rähse, Josef Steber, Frederike Wiebel, Anette Nordskog, "Laundry Detergents" in Ullmann’s Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_315.pub2
- ^ Commoner, Barry (1971). The Closing Circle: Nature, Man, and Technology. New York, NY: Random House. ISBN 039442350X.
- ^ a b Outwater, Alice (1996). Water: A Natural History. New York, NY: Basic Books. p. 155. ISBN 0-465-03780-1.
- ^ "Detergents Guidance Document". Health and Safety Executive. http://www.detergents.gov.uk/detergents.asp?id=1647. Retrieved 4 March 2011.
- ^ "Detergents Home Page". Health and Safety Executive. http://www.detergents.gov.uk/detergents_home.asp. Retrieved 4 March 2011.
- ^ Barlass, Tim (12 June 2011). "Detergents to dump phosphates". The Sydney Morning Herald. http://www.smh.com.au/environment/detergents-to-dump-phosphates-20110611-1fxzm.html. Retrieved 15 June 2011. "He is now in discussions with a detergent industry group about retiring the P phosphate symbol once the full ban starts in 2014."
- ^ "Reducing Wastewater Salinity from Detergents". Ministry of the Environment (Israel). http://www.sviva.gov.il/Enviroment/Static/Binaries/Articals/Deterg~1_0.pdf. Retrieved 15 June 2011.
External links
- Derbyshire, David (24 February 2008). "Don't bother with pre-wash (you're just wasting 6billion litres of water a year)". Mail Online. http://www.dailymail.co.uk/news/article-518457/Dont-bother-pre-wash-youre-just-wasting-6billion-litres-water-year.html. Retrieved 30 September 2010.
- http://housewares.about.com/od/laundryappliances/f/hedetergents.htm
- http://www.chemistry.co.nz/introduction.htm
- http://www.laundryworks.ca//soap.com
Categories:- Laundry detergents
Wikimedia Foundation. 2010.