- Isotopes of neptunium
-
Neptunium (Np) is an artificial element, and thus a standard atomic mass cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 239Np in 1940, produced by bombarding 238U with neutrons to produce 239U, which then underwent beta decay to 239Np.
Trace quantities are found in nature from neutron capture by uranium atoms.
Twenty neptunium radioisotopes have been characterized, with the most stable being 237
Np with a half-life of 2.14 million years, 236
Np with a half-life of 154,000 years, and 235
Np with a half-life of 396.1 days. All of the remaining radioactive isotopes have half-lives that are less than 4.5 days, and the majority of these have half-lives that are less than 50 minutes. This element also has 4 meta states, with the most stable being 236m
Np (t½ 22.5 hours).The isotopes of neptunium range in atomic weight from 225.0339 u (225
Np) to 244.068 u (244
Np). The primary decay mode before the most stable isotope, 237
Np, is electron capture (with a good deal of alpha emission), and the primary mode after is beta emission. The primary decay products before 237
Np are isotopes of uranium and protactinium, and the primary products after are isotopes of plutonium.Contents
Some notable isotopes
Neptunium-235
Neptunium-235 has 142 neutrons and a half-life of 400 days. This isotope of Neptunium either decays by:
- Emitting an alpha particle - Here, the decay energy is 5.2 MeV and the decay product is Protactinium-231.
- Electron capture - Here, the decay energy is 0.125 MeV and the decay product is Uranium-235
This particular isotope of neptunium has a weight of 235.0440633 grams/mole.
Neptunium-236
Neptunium-236 has 143 neutrons and a half-life of 154,000 years. It can decay by the following methods -
- Electron capture - here, the decay energy is 0.95 MeV and the decay product is Uranium-236.
- Beta emission - Here, the decay energy is 0.94 MeV and the decay product is Plutonium-236.
- Alpha emission - Here, the decay energy is 5.024 MeV and the decay product is Protactinium-232
This particular isotope of neptunium has a mass of 236.04657 grams/mole. It is a fissile material with a critical mass of 7 kg.
possible parent nuclides: alpha from Am-240
Neptunium-237
Actinides Half-life Fission products 244Cm 241Pu f 250Cf 243Cmf 10–30 y 137Cs 90Sr 85Kr 232U f 238Pu f is for
fissile69–90 y 151Sm nc➔ 4n 249Cf f 242Amf 141–351 No fission product
has half-life 102
to 2×105 years241Am 251Cf f 431–898 240Pu 229Th 246Cm 243Am 5–7 ky 4n 245Cmf 250Cm 239Pu f 8–24 ky 233U f 230Th 231Pa 32–160 4n+1 234U 4n+3 211–290 99Tc 126Sn 79Se 248Cm 242Pu 340–373 Long-lived fission products 237Np 4n+2 1–2 My 93Zr 135Cs nc➔ 236U 4n+1 247Cmf 6–23 My 107Pd 129I 244Pu 80 My >7% >5% >1% >.1% 232Th 238U 235U f 0.7–12 Ty fission product yield 237
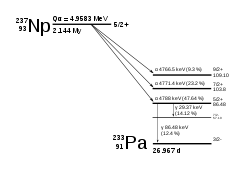
Np decays via the neptunium series to thallium, unlike most other actinides which decay to isotopes of lead.237
Np was recently shown to be capable of sustaining a chain reaction with fast neutrons, as in a nuclear weapon.[1] However, it has a low probability of fission on bombardment with thermal neutrons, which makes it unsuitable as a fuel for nuclear power plants.237
Np is the only neptunium isotope produced in significant quantity in the nuclear fuel cycle, both by successive neutron capture on uranium-235 (which fissions most but not all of the time) and uranium-236, or (n,2n) reactions where a fast neutron occasionally knocks a neutron loose from uranium-238 or isotopes of plutonium. Over the long term, 237
Np also forms in spent nuclear fuel as the decay product of americium-241.237
Np is projected to be one of the most mobile nuclides at the Yucca Mountain nuclear waste repository.Table
nuclide
symbolZ(p) N(n)
isotopic mass (u)
half-life decay
mode(s)[2][n 1]daughter
isotope(s)nuclear
spinexcitation energy 225
Np93 132 225.03391(8) 3# ms [>2 µs] α 221Pa 9/2-# 226
Np93 133 226.03515(10)# 35(10) ms α 222Pa 227
Np93 134 227.03496(8) 510(60) ms α (99.95%) 223Pa 5/2-# β+ (.05%) 227U 228
Np93 135 228.03618(21)# 61.4(14) s β+ (59%) 228U α (41%) 224Pa β+, SF (.012%) (various) 229
Np93 136 229.03626(9) 4.0(2) min α (51%) 225Pa 5/2+# β+ (49%) 229U 230
Np93 137 230.03783(6) 4.6(3) min β+ (97%) 230U α (3%) 226Pa 231
Np93 138 231.03825(5) 48.8(2) min β+ (98%) 231U (5/2)(+#) α (2%) 227Pa 232
Np93 139 232.04011(11)# 14.7(3) min β+ (99.99%) 232U (4+) α (.003%) 228Pa 233
Np93 140 233.04074(5) 36.2(1) min β+ (99.99%) 233U (5/2+) α (.001%) 229Pa 234
Np93 141 234.042895(9) 4.4(1) d β+ 234U (0+) 235
Np93 142 235.0440633(21) 396.1(12) d EC 235U 5/2+ α (.0026%) 231Pa 236
Np93 143 236.04657(5) 1.54(6)×105 a EC (87.3%) 236U (6-) β- (12.5%) 236Pu α (.16%) 232Pa 236m
Np60(50) keV 22.5(4) h EC (52%) 236U 1 β- (48%) 236Pu 237
Np[n 2][n 3]93 144 237.0481734(20) 2.144(7)×106 a α 233Pa 5/2+ SF (2×10−10%) (various) CD (4×10−12%) 207Tl
30Mg238
Np93 145 238.0509464(20) 2.117(2) d β- 238Pu 2+ 238m
Np2300(200)# keV 112(39) ns 239
Np93 146 239.0529390(22) 2.356(3) d β- 239Pu 5/2+ 240
Np93 147 240.056162(16) 61.9(2) min β- 240Pu (5+) 240m
Np20(15) keV 7.22(2) min β- (99.89%) 240Pu 1(+) IT (.11%) 240Np 241
Np93 148 241.05825(8) 13.9(2) min β- 241Pu (5/2+) 242
Np93 149 242.06164(21) 2.2(2) min β- 242Pu (1+) 242m
Np0(50)# keV 5.5(1) min 6+# 243
Np93 150 243.06428(3)# 1.85(15) min β- 243Pu (5/2-) 244
Np93 151 244.06785(32)# 2.29(16) min β- 244Pu (7-) - ^ Abbreviations:
CD: Cluster decay
EC: Electron capture
IT: Isomeric transition
SF: Spontaneous fission - ^ Fissile nuclide
- ^ Most common nuclide
Notes
- Values marked # are not purely derived from experimental data, but at least partly from systematic trends. Spins with weak assignment arguments are enclosed in parentheses.
- Uncertainties are given in concise form in parentheses after the corresponding last digits. Uncertainty values denote one standard deviation, except isotopic composition and standard atomic mass from IUPAC which use expanded uncertainties.
References
- ^ P. Weiss (26 October 2002). "Little-studied metal goes critical - Neptunium Nukes?". Science News. http://www.findarticles.com/p/articles/mi_m1200/is_17_162/ai_94011322. Retrieved 2006-09-29.
- ^ http://www.nucleonica.net/unc.aspx
- Isotope masses from:
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties". Nuclear Physics A 729: 3–128. Bibcode 2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001. http://www.nndc.bnl.gov/amdc/nubase/Nubase2003.pdf.
- Isotopic compositions and standard atomic masses from:
- J. R. de Laeter, J. K. Böhlke, P. De Bièvre, H. Hidaka, H. S. Peiser, K. J. R. Rosman and P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry 75 (6): 683–800. doi:10.1351/pac200375060683. http://www.iupac.org/publications/pac/75/6/0683/pdf/.
- M. E. Wieser (2006). "Atomic weights of the elements 2005 (IUPAC Technical Report)". Pure and Applied Chemistry 78 (11): 2051–2066. doi:10.1351/pac200678112051. http://iupac.org/publications/pac/78/11/2051/pdf/. Lay summary.
- Half-life, spin, and isomer data selected from the following sources. See editing notes on this article's talk page.
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties". Nuclear Physics A 729: 3–128. Bibcode 2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001. http://www.nndc.bnl.gov/amdc/nubase/Nubase2003.pdf.
- National Nuclear Data Center. "NuDat 2.1 database". Brookhaven National Laboratory. http://www.nndc.bnl.gov/nudat2/. Retrieved September 2005.
- N. E. Holden (2004). "Table of the Isotopes". In D. R. Lide. CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. Section 11. ISBN 978-0849304859.
Isotopes of uranium Isotopes of neptunium Isotopes of plutonium Index to isotope pages · Table of nuclides Categories:- Neptunium
- Isotopes of neptunium
- Lists of isotopes by element
Wikimedia Foundation. 2010.