- Acetic oxalic anhydride
-
Acetic oxalic anhydride 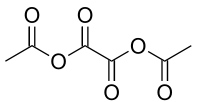
Identifiers CAS number 19037-85-5 ChemSpider 25991421 Jmol-3D images Image 1 - CC(OC(C(OC(C)=O)=O)=O)=O
- InChI=1S/C6H6O6/c1-3(7)11-5(9)6(10)12-4(2)8/h1-2H3
Key: XRMBFFGBVGXJJF-UHFFFAOYSA-N
Properties Molecular formula C6H6O6 Molar mass 174.11 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Acetic oxalic anhydride is an organic compound with formula C6H6O6 or (H3C-(C=O)-O-(C=O)-)2. It can be viewed as a mixed anhydride, formally derived from acetic acid (H3C-(C=O)OH) and oxalic acid ((-(C=O)OH)2), in 2:1 molecular ratio, by the loss of two water molecules.
It is an unstable colorless crystalline solid, soluble in diethyl ether, that decomposes at about -3 °C into acetic anhydride (H3C-(C=O)-)2O, carbon dioxide (CO2) and carbon monoxide (CO). It is hydrolyzed by water into acetic and oxalic acids.[1]
Unlike some other anhydrides, it cannot be obtained directly from the acids. It was synthesized in 1953 by W. Edwards and W. M. Henley, by reacting silver oxalate (Ag2C2O4) suspended in diethyl ether with acetyl chloride at temperatures below -5°C and distilling off the solvent under low pressure. It can also be obtained by reacting anhydrous oxalic acid with ketene (H2C=O=O).[1]
Acetic oxalic anhydride was conjectured to be an intermediate in the decomposition of anhydrous oxalic acid by acetic anhydride.[1]
See also
References
- ^ a b c W. R. Edwards and Walter M. Henley (1953), Acetic Oxalic Anhydride. J. Am. Chem. Soc., volume 75, issue 14, pages 3857-3859. doi:10.1021/ja01110a505
Categories:- Acid anhydrides
Wikimedia Foundation. 2010.
