- Octyl glucoside
-
β-D-Octyl glucoside 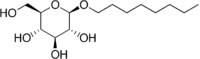 (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-octoxyoxane-3,4,5-triolOther namesn-octyl-β-D-glucoside
(2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-octoxyoxane-3,4,5-triolOther namesn-octyl-β-D-glucosideIdentifiers CAS number 29836-26-8 
PubChem 62852 Jmol-3D images Image 1 - CCCCCCCCO[C@H]1[C@@H]([C@H]([C@@H]([C@H](O1)CO)O)O)O
Properties Molecular formula C14H28O6 Molar mass 292.37 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Octyl glucoside (n-octyl-β-D-glucoside) is a detergent frequently used to dissolve integral membrane proteins for studies in biochemistry. Like Genapol X-100 and Triton X-100, it is a nonphysiological amphiphile that makes lipid bilayers less "stiff".[1]
It has become one of the most important detergents for purification of membrane proteins because it can readily be removed from final protein extracts.[2] Above its critical micelle concentration of 0.7%, it was noted as the best detergent for improving selectivity of immunoprecipitation of phosphotyrosine modified proteins.[3]
The compound gained popularity with researchers following the publication of an improved synthesis in 1978.[4][5] However, in 1990 the cost remained prohibitive for large-scale protein isolation.[6]
Octyl glucoside has been proposed as a conditioning agent to prevent microbial colonization of contact lenses, due to its ability to lower the hydrophobicity of contact lenses and prevent adhesion of Staphylococcus epidermidis and Pseudomonas aeruginosa.[7]
See also
References
- ^ Lundbaek, Ja; Birn, P; Hansen, Aj; Søgaard, R; Nielsen, C; Girshman, J; Bruno, Mj; Tape, Se; Egebjerg, J; Greathouse, Dv; Mattice, Gl; Koeppe, Re, 2Nd; Andersen, Os (May 2004). "Regulation of Sodium Channel Function by Bilayer Elasticity: The Importance of Hydrophobic Coupling. Effects of Micelle-forming Amphiphiles and Cholesterol" (Free full text). The Journal of general physiology 123 (5): 599–621. doi:10.1085/jgp.200308996. ISSN 0022-1295. PMC 2234500. PMID 15111647. http://www.jgp.org/cgi/pmidlookup?view=long&pmid=15111647.
- ^ Morandat, S; El, Kirat, K (Apr 2007). "Solubilization of supported lipid membranes by octyl glucoside observed by time-lapse atomic force microscopy". Colloids and surfaces. B, Biointerfaces 55 (2): 179–84. doi:10.1016/j.colsurfb.2006.11.039. ISSN 0927-7765. PMID 17207975.
- ^ Zhang, G; Neubert, Ta (Jan 2006). "Use of detergents to increase selectivity of immunoprecipitation of tyrosine phosphorylated peptides prior to identification by MALDI quadrupole-TOF MS". Proteomics 6 (2): 571–8. doi:10.1002/pmic.200500267. ISSN 1615-9853. PMID 16342243.
- ^ See PubMed search for "octyl[Title] AND glucoside[Title]" for a timeline of publications.
- ^ Keana, Jf; Roman, Rb (1978). "Improved synthesis of n-octyl-beta-D-glucoside: a nonionic detergent of considerable potential in membrane biochemistry". Membrane biochemistry 1 (3–4): 323–7. ISSN 0149-046X. PMID 756493.
- ^ Kobs, Sf (Nov 1990). "Recovery of octyl beta-glucoside from detergent/protein mixtures". Analytical biochemistry 191 (1): 47–9. doi:10.1016/0003-2697(90)90385-M. ISSN 0003-2697. PMID 2077942.
- ^ Santos, L; Rodrigues, D; Lira, M; Oliveira, R; Real, Oliveira, Me; Vilar, Ey; Azeredo, J (May 2007). "The effect of octylglucoside and sodium cholate in Staphylococcus epidermidis and Pseudomonas aeruginosa adhesion to soft contact lenses". Optometry and vision science : official publication of the American Academy of Optometry 84 (5): 429–34. doi:10.1097/OPX.0b013e318058a0cc. ISSN 1040-5488. PMID 17502827.
Categories:- Glucosides
- Non-ionic surfactants
Wikimedia Foundation. 2010.
