- Neodymium(III) oxide
-
Neodymium(III) oxide 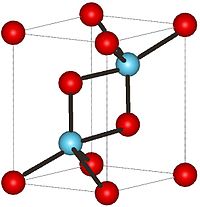
 Neodymium(III) oxideOther namesNeodymium oxide, Neodymium sesquioxide
Neodymium(III) oxideOther namesNeodymium oxide, Neodymium sesquioxideIdentifiers CAS number 1313-97-9 
Properties Molecular formula Nd2O3 Molar mass 336.48 g/mol Appearance light bluish gray hexagonal crystals Density 7.24 g/cm3 Melting point 2233°C
Boiling point 3760°C[1]
Solubility in water .0003 g/100 mL (75 °C) Structure Crystal structure Hexagonal, hP5 Space group P-3m1, No. 164 Thermochemistry Std enthalpy of
formation ΔfHo298-1807.9 kJ·mol-1 Standard molar
entropy So298158.6 J·mol-1·K-1 Specific heat capacity, C 111.3 J·mol-1·K-1[1] Related compounds Other anions Neodymium(II) chloride
Neodymium(III) chlorideOther cations Uranium(VI) oxide
Praseodymium(III) oxide
Promethium(III) oxide (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Neodymium(III) oxide or neodymium sesquioxide is the chemical compound composed of neodymium and oxygen with the formula Nd2O3. It forms very light grayish blue hexagonal crystals[1]. The rare earth mixture didymium, previously believed to be an element, partially consists of neodymium(III) oxide[2].
Uses
Neodymium(III) oxide is used to dope glass, including sunglasses, make solid-state lasers, and to color glasses and enamels[3]. Neodymium-doped glass turns purple due to the absorbance of yellow and green light, and is used in welding goggles[4]. Some neodymium-doped glass is dichroic; that is, it changes color depending on the lighting. One kind of glass named for the mineral alexandrite appears blue in sunlight and red in artificial light[5]. 7000 tonnes of neodymium(III) oxide are produced worldwide each year. Neodymium(III) oxide is also used as a polymerization catalyst[4].
Reactions
Neodymium(III) oxide is formed when neodymium(III) nitride or neodymium(III) hydroxide is burned in air[6].
References
- ^ a b c Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 471; 552, ISBN 0849305942
- ^ Brady, George Stuart; Clauser, Henry R.; Vaccari, John A. (2002), Materials Handbook (15 ed.), New York: McGraw-Hill Professional, pp. 779, ISBN 9780071360760, http://books.google.com/?id=vIhvSQLhhMEC&pg=PA779&dq=%22Neodymium+oxide%22, retrieved 2009-03-18
- ^ Eagleson, Mary (1994), Concise Encyclopedia of Chemistry, Springer, pp. 680, ISBN 9783110114515, http://books.google.com/?id=Owuv-c9L_IMC&pg=PA680&dq=%22Neodymium(III)+oxide%22, retrieved 2009-03-18
- ^ a b Emsley, John (2003), Nature's Building Blocks, Oxford University Press, pp. 268–9, ISBN 9780198503408, http://books.google.com/?id=j-Xu07p3cKwC&pg=PA268&dq=%22Neodymium+oxide%22, retrieved 2009-03-18
- ^ Bray, Charles (2001), Dictionary of Glass (2 ed.), University of Pennsylvania Press, pp. 103, ISBN 9780812236194, http://books.google.com/?id=KbZkxDyeG18C&pg=PA102&dq=%22Neodymium+oxide%22, retrieved 2009-03-18
- ^ Spencer, James Frederick (1919), The Metals of the Rare Earths, London: Longmans, Green, and Co, pp. 115, http://books.google.com/?id=W2zxN_FLQm8C&pg=PA115&dq=%22Neodymium+oxide%22, retrieved 2009-03-18
Categories:- Neodymium compounds
- Oxides
- Sesquioxides
Wikimedia Foundation. 2010.
