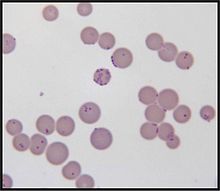
- M. haemofelis
-
Mycoplasma haemofelis Scientific classification Kingdom: Bacteria Phylum: Tenericutes Class: Mollicutes Order: Mycoplasmatales Family: Mycoplasmataceae Genus: Mycoplasma
Nowak 1929Species: M. haemofelis Binomial Name
Mycoplasma haemofelis[1]
Mycoplasma haemofelis (formerly Haemobartonella felis) is a gram negative epierythrocytic parasitic bacterium. It often appears in bloodsmears as small (0.6μM) coccoid bodies, sometimes forming short chains of 3 to 6 organisms. It is usually the causative agent of Feline Infectious Anemia (FIA) in the United States.[2]
The ~1200kb genome contains a minimalistic assortment of genes limited to the most basic cellular functions.[3] This leaves M. haemofelis inextricably dependent upon its host for the provision of amino acids, cholesterol, vitamins, and fatty acids. The complex and specific conditions the bacterium requires have made it impossible to culture outside a host thus far.[4]
Arthropod vectors are thought to be the primary source of infection, although M. haemofelis is also known to be transmitted from queen to kitten and following blood transfusion. Immunocompromization and/or coinfection with FeLV, FIV, and other Mycoplasma species can exacerbate symptoms or cause symptoms to arise in previously asymptomatic individuals. Symptoms include anemia, lethargy, fever, and anorexia.[5]
In suspected cases, M. haemofelis can be identified by polymerase chain reaction analysis for species-specific 16S rRNA sequences as well as by light microscopy. Treatment usually includes the administration of doxycycline or enrofloxacin to quell the infection along with transfusion and administration of glucocorticoids to alleviate anemia.[5]
Recent evidence suggests that M. haemofelis may be transmissible to humans.[6]
Contents
Classification
M. haemofelis belongs to the phylogenetically diverse class Mollicutes, which comprises 8 genera: Ureaplasma, Spiroplasma, Asteroleplasma, Mesoplasma, Entomoplasma, Acholeplasma, Anaeroplasma, and Mycoplasma. Haemoplasmas is the name given to the trivial cluster that includes M. haemofelis and its close relatives.[2]
Before the advent of modern PCR techniques, M. haemofelis and closely related Haemoplasmas Candidatus Mycoplasma haemominutum and Candidatus Mycoplasma turicensis were collectively classified as Haemobartonella felis based on similarities in gross morphology. The Candidatus distinction is given to newly described species in which additional evidence is required to support their classification. The inability of researchers to culture many Mycoplasma spp. in vitro has made classification difficult. PCR analysis of 16S rRNA sequences of Haemobartonella spp. showed greater similarity to those of Mollicutes than to those of the family Anaplasmataceae in the order Rickettsiales to which they were previously thought to belong.[7]
PCR-based assays have provided evidence that the Ohio variant and California variant of H. felis are in fact distinct species, M. haemofelis and Candidatus Mycoplasma haemominutum respectively. A third Haemoplasma, Mycoplasma turicensis, was later identified in domestic cats. Haemoplasma species have also been identified in dogs (M. haemocanis), mice (M. haemomuris), opossum (Candidatus M. haemodidelphis), and alpaca (Candidatus M. haemolamae). In cats, M. haemofelis is the most virulent Haemoplasma species and is most often implicated in FIA in the United States.[8]
Transmission
Although M. haemofelis is generally the least prevalent of the three known feline Haemoplasmas, it causes the majority of FIA cases in the United States. Blood-sucking Arthropod vectors including fleas, mosquitoes, and ticks are thought to be the primary mode of dissemination of M. haemofelis.[5] Transmission from queen to kitten has also been observed, however it is unclear if this takes place in utero, during birth, or through nursing. M. haemofelis has been transmitted by transfusion and oral administration of infected blood. Males show a significant disposition towards M. haemofelis infection. It is thought that biting and scratching may result in the infection of toms involved in aggressive behavior.[4]
Pathogenesis
Through reductive evolution, the average genome size of M. haemofelis has been decreased to 1245kb.[9] It has shed many biosynthetic systems found in related gram-positive bacteria as well as the ability to secrete a cell wall (rendering it technically gram-negative). This reduction of genetic information has committed M. haemofelis to a parasitic lifestyle in which it is entirely dependent upon host cells for the amino acids, fatty acids, and vitamins it has lost the ability to synthesize. Consistent with its parasitic lifestyle, the M. haemofelis genome contains a significant number of genes devoted to adhesins, resistance to oxidative stress, and the production of variable surface antigens that allow it to persist in the host.[4]
Once in the blood stream, M. haemofelis individuals adhere to the cell membranes of red blood cells and eventually become partially embedded. After a delay of 2 to 34 days, the acute phase of infection occurs during which marked parasitemia is often observed. In some cases, up to 90% of red blood cells become parasitized. During this stage of infection that M. haemofelis organisms can be identified in a stained blood smear under light microscopy.[5]
Synchronous phase variation has been observed in natural M. haemofelis infections during which rapid fluctuations in parasitemia are observed. This spontaneous alteration of phenotype seems to allow individuals to detach from erythrocytes by the alteration or concealment of surface antigens. This may facilitate the persistence of M. haemofelis within the host by disguising or eliminating antigens that might illicit an immune response. [10]
Parasitized red blood cells often lose their biconcave shape. This decreases surface area, increases osmotic fragility, and increases the likelihood that these cells will be captured and destroyed by the spleen. The attachment of M. haemofelis to red blood cell membranes is often associated with positive Coombs test results, meaning IgG antibodies have become bound to red blood cells, marking them for destruction. For the most part, the anemia seen in M. haemofelis infection is a result of extravascular erythrophagocytosis by macrophages in the spleen, liver, lungs, and bone marrow.[5]
Left untreated, as many as one third of cats with acute M. haemofelis infection will die from severe anemia. In cats that mount adequate immune and regenerative responses to acute infection, a recovery time of a month or more may be required before the hematocrit returns to normal. During this recovery time,M. haemofelis is still often observed in circulating blood but in decreased numbers. Cats that recover from acute infections may remain infected for life.[5] Intact M. haemofelis organisms have been observed in the phagocytic vacuoles of splenic and pulmonary macrophages, suggesting that these cells may serve as reservoirs.[4]
Co-infection with FIV, FeLV, and Candidatus Mycoplasma haemominutum is common. Although M. haemofelis infection can cause acute hemolytic anemia in otherwise healthy cats, immunosuppression, including that brought on by retroviral pathogenesis, increases susceptibility to the most severe effects of M. haemofelis infection. In some cases, infected cats may remain asymptomatic carriers until compromisation of the immune system permits increased parasitemia and the onset of acute symptoms. Interestingly, chronic M. haemofelis infection may promote neoplastic transformation of white blood cells in FeLV-infected individuals,.[5][11]
Diagnosis
The severity of disease produced by M. haemofelis varies, with some cats having mild anemia and no clinical signs to cats having marked depression and severe anemia. Clinical signs include lethargy, anorexia, and anemia. M. haemofelis infection is suspected in cats with regenerative anemia, in which polychromasia and reticulocytosis are noted. During the acute phase of infection, M. haemofelis can be readily identified on stained blood films however M. haemofelis can disappear and reappear in the peripheral blood throughout the course of infection and can be mistaken for stain precipitate or vice versa. Commercially available PCR assays that detect the Mycoplasma 16s rRNA are more reliable means of diagnosis. Many such assays are species specific. Currently, no serological test for M. haemofelis is commercially available. Additional clinical findings may include positive Coombs test results, hypoglycemia, and dehydration.[5]
Treatment
Antibiotic treatment is indicated only for Haemoplasma-positive cats that present clinical signs for FIA. While it is not believed that M. haemofelis can completely eliminated, regimens of doxycycline or enrofloxacin are effective in reducing parasitemia. Doxycycline and enrofloxacin combat M. haemofelis infection by interfering with translation and DNA synthesis respectively. These antibiotics carry side effects including esophagitis, GI disease, and retinal damage and are thus primarily administered only to cats suffering from acute infection with clinical signs.[12] Furthermore, blood transfusion and administration of glucocorticoids relieve the severe anemia resulting from M. haemofelis infection of erythrocytes. Treated and untreated animals that recover from M. haemofelis infections generally remain carriers but seldom relapse with clinical disease.[5]
Public Health Implications
Arthropod vectors appear to be the primary mechanism of M. haemofelis transmission.[5] Mycoplasma DNA sequences have been detected in fleas, ticks, and mosquitoes.[4] Given that humans often cohabitate with cats and that species of blood-sucking arthropods inhabit most temperate regions, transmission of Haemoplasmas to humans appears possible. Furthermore, all 3 feline Haemoplasma species have been detected in wild felids, suggesting the possibility that they may act as reservoirs of infection for arthropod transmission.[13] In 2008, M. haemofelis was detected in an AIDS patient from Brazil.[6] The zoonotic potential of M. haemofelis has yet to be fully accessed, but care should be taken when handling blood or tissues from infected cats.[2]
References
- ^ Garrity, edited by George (2008). Bergey's manual of systematic bacteriology. (2nd ed. ed.). New York: Springer. ISBN 0-387-95041-9.
- ^ a b c Messick, Joanne B. (2004). "Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential". Veterinary Clinical Pathology 33 (1): 2–13.
- ^ Razin, S (1992). "Peculiar properties of mycoplasmas: the smallest self-replicating prokaryotes". FEMS Microbiol Lett 100: 423–432.
- ^ a b c d e Sykes, Jane E. (1 November 2010). "Feline Hemotropic Mycoplasmas". Veterinary Clinics of North America: Small Animal Practice 40 (6): 1157–1170. doi:10.1016/j.cvsm.2010.07.003.
- ^ a b c d e f g h i j Greene, [edited by] Craig E. (2006). Infectious diseases of the dog and cat (3rd ed. ed.). St. Louis, Mo.: Saunders Elsevier. ISBN 978-1-4160-3600-5.
- ^ a b Santos, AP; Santos RP, Biondo AW, et al. (2009). "Hemoplasma infection in an HIV positive patient, Brazil". Emerg Infect Dis 14: 1922–1924.
- ^ Neimark, H; Johanasson KE, Rikihisa Y, Tully JG (2001). "Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of Candidatus M. haemofelis, Candidatus M. haemomuris, Candidatus M. haemosuis and Candidatus M. wenyonii". Int J Syst Evol Microbiol 51: 891–899.
- ^ Messick, JB; Walker PG, Raphel W, Berent L, Shi X (2002). "Candidatus M. haemodidelphis' sp. nov., Candidatus M. haemolamae sp. nov., and M. haemocanis comb. nov., haemotrophic parasites from a naturally infected opossum, alpaca, and dog: phylogenic and secondary structural relatedness of their 16S rRNA genes to other mycoplasmas". Int J Syst Evol Microbiol 52: 689–693.
- ^ Berent, LM; Messick JB (2003). "Physical map and genome sequencing survey of Mycoplasma haemofelis". Infect Immun. 71: 3657–3662.
- ^ Berent, LM; Messick JB, Cooper SK (1998). "Detection of Haemobartonella felis in cats with experimentally induced acute and chronic infections using a PCR assay". Am J Vet Rev 59: 1215–1220.
- ^ Bobade, PA; Nash AS, Rogerson P (1988). "Feline haemobaronellosis: clinical, haemotological and pathological studies in natural infections and relationship to infection with feline leukaemia virus". Vet Rec 122: 32–36.
- ^ Tasker, S; Helps CR, Day MJ (2004). "Use of a Taqman PCR to determine the response of M. haemofelis infection to antibiotic treatment". J Microbiol Methods 56: 63–71.
- ^ Willi, B; Filoni C, Catao-Diaz JL (2007). "Worldwide occurrence of feline haemoplasma infections in wild felid species". J Clin Microbiol 45 (4): 1159–1166.
Categories:
Wikimedia Foundation. 2010.