- Sodium ascorbate
-
Sodium L-ascorbate[1] 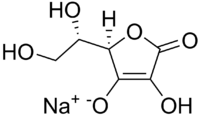 Sodium (2R)-2-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2H-furan-3-olateOther namesSodascorbate; Monosodium ascorbate; E301
Sodium (2R)-2-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2H-furan-3-olateOther namesSodascorbate; Monosodium ascorbate; E301Identifiers CAS number 134-03-2 
PubChem 23667548 ChEMBL CHEMBL1201176 
Jmol-3D images Image 1 - OC1=C([O-])[C@]([C@@H](O)CO)([H])OC1=O.[Na+]
Properties Molecular formula C6H7NaO6 Molar mass 198.11 g mol−1 Melting point 220 °C (dec.)
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium ascorbate is a more bioavailable form of vitamin C that is an alternative to taking ascorbic acid as a supplement. The molecular formula of this chemical compound is C6H7NaO6. As the sodium salt of ascorbic acid (vitamin C), it is known as a mineral ascorbate.
Sodium ascorbate normally provides 131 mg of sodium per 1,000 mg of ascorbic acid (1,000 mg of sodium ascorbate contains 889 mg of ascorbic acid and 111 mg of sodium).
As a food additive, it has the E number E301 and is used as an antioxidant and an acidity regulator. It is approved for use as a food additive in the EU[2], USA [3] and Australia and New Zealand.[4]
Sodium ascorbate can reverse the development of atherosclerotic disease, helps in heart attack prevention.[5]In addition sodium ascorbate plays a significant role in the elimination of chronic and acute infections.[6] Moreover, it is considered to be an anti-cancer agent. Sodium ascorbate produces cytotoxic effect in an array of malignant cell lines, which include melanoma cells that are particularly susceptible. [7] [8]
Side-effects of sodium ascorbate
While sodium ascorbate is relatively safe, its extensive intake may lead to several side-effects. The common side-effects that persist or become troublesome when using sodium ascorbate, especially in a liquid form as injections, include burning, stinging, pain, or swelling at the injection site. Severe allergic reactions include rash; itching; difficult breathing; hives; tightness in the chest; swelling of the mouth, face, lips, or tongue; bone pain; muscle weakness; severe or persistent diarrhea; mental or mood changes. [9]
References
- ^ (+)-Sodium L-ascorbate at Sigma-Aldrich
- ^ UK Food Standards Agency: "Current EU approved additives and their E Numbers". http://www.food.gov.uk/safereating/chemsafe/additivesbranch/enumberlist. Retrieved 2011-10-27.
- ^ US Food and Drug Administration: "Listing of Food Additives Status Part II". http://www.fda.gov/Food/FoodIngredientsPackaging/FoodAdditives/ucm191033.htm#ftnT. Retrieved 2011-10-27.
- ^ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". http://www.comlaw.gov.au/Details/F2011C00827. Retrieved 2011-10-27.
- ^ Chiara Arrigonia, Davide Camozzia, Barbara Imbertia, Sara Manterob and Andrea Remuzzia (February 2006). "The effect of sodium ascorbate on the mechanical properties of hyaluronan-based vascular constructs". Biomaterials 27 (4): 623–630. doi:10.1016/j.biomaterials.2005.06.009. PMID 16048730.
- ^ Deanna Arquette, Linda D. Caren (1992). "Effect of sodium ascorbate on the chemiluminescent response of murine peritoneal exudate cells". Life Sciences 50 (11): 753–759. doi:10.1016/0024-3205(92)90179-S. PMID 1740959.
- ^ Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. (September 2005). "Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues.". Proc Natl Acad Sci U S A. 102 (38): 13604–9. doi:10.1073/pnas.0506390102. PMC 1224653. PMID 16157892. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1224653.
- ^ Kang JS, Cho D, Kim YI, Hahm E, Kim YS, Jin SN, Kim HN, Kim D, Hur D, Park H, Hwang YI, Lee WJ. . (July 2005). "Sodium ascorbate (vitamin C) induces apoptosis in melanoma cells via the down-regulation of transferrin receptor dependent iron uptake.". J Cell Physiol. 204 (1): 192–7. doi:10.1002/jcp.20286. PMID 15672419.
- ^ Frederick R. Klenner, Lendon H. Smith (October 1991). Clinical Guide to the Use of Vitamin C: The Clinical Experiences of Frederick R. Klenner, M.D. (Clinical Guide to the Use of Vitamin C: Abbreviated, Summarized and Annotated by Lendon H. Smith, M.D. ed.). Tacoma, Wash: Life Sciences Press. ISBN 9780943685137. http://www.seanet.com/~alexs/ascorbate/198x/smith-lh-clinical_guide_1988.htm.
External links
Categories:- Ascorbates
- Food additives
- Sodium compounds
- Vitamers
- Vitamin C
Wikimedia Foundation. 2010.
