- 2-Methyltetrahydrofuran
-
2-Methyltetrahydrofuran 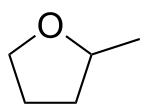
Identifiers CAS number 96-47-9 
ChemSpider 7028 
Jmol-3D images Image 1 - O1C(C)CCC1
Properties Molecular formula C5H10O Molar mass 86.13 g mol−1 Density 0.854 g/mL Melting point -136 °C, 137 K, -213 °F
Boiling point 80.3 °C, 353 K, 177 °F
Hazards MSDS External MSDS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2-Methyltetrahydrofuran is an organic compound with the molecular formula CH3C4H7O. It is a highly flammable mobile liquid. It is mainly used as a replacement for THF in specialized applications for its better performance, such as to obtain higher reaction temperatures, or easier separations due to the solubility of 2-methyltetrahydrofuran. It is derived from furfural and is usable as a biofuel.
Contents
Structures and properties
2-Methyltetrahydrofuran is “inversely soluble” in water. That is, its solubility decreases with increasing temperature, which is a rare property.[1] 2-Methyltetrahydrofuran behaves like tetrahydrofuran as a Lewis base in organometallic reactions.[2]
Preparation
2-Methyltetrahydrofuran is usually synthesized by catalytic hydrogenation of furfural.
- OC4H3CHO + 4 H2 → OC4H7CH3 + H2O
Furfural is produced by the acid-catalyzed digestion of pentosan sugars, C5 polysaccharides, in biomass. Thus, the raw materials of 2-methyltetrahydrofuran are renewable biomass rich with cellulose, hemicelluloses, and lignin, such as corncobs or bagasse and other plant and agricultural waste.[3]
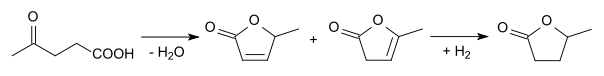
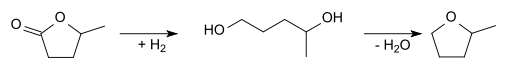
2-Methyltetrahydrofuran can also be produced starting from levulinic acid. Cyclization and reduction gives γ-valerolactone:
This lactone can be hydrogenated to 1,4-pentanediol, which can then be dehydrated to give 2-methyltetrahydrofuran:
2-Methyltetrahydrofuran has one stereocenter, so it exists in two enantiomeric forms. The commercial processes involving hydrogenation gives a racemic mixture of the two. The asymmetric synthesis of (S)-(+)-2-methyltetrahydrofuran can achieved by using a wool–rhodium complex as a chiral catalyst for hydrogenation of methyl furan.[1]
Applications
2-Methyltetrahydrofuran is mainly used as a higher boiling substitute for tetrahydrofuran as specialty solvent. It also is used in the electrolyte formulation for secondary lithium electrodes and as a component in alternative fuels. It is a valued solvent for low temperature reactions. 2-Methyltetrahydrofuran forms a glass, which does not crystallize, and is frequently used as a solvent for spectroscopic studies at -196 °C. [2]
Other common uses of 2-methyltetrahydrofuran is as a solvent for Grignard reagents used in organometallic and biphasic chemical processes, because of the oxygen atom's ability to coordinate to the magnesium ion component of the Grignard reagent, or to azeotropically dry products. The use of 2-methyltetrahydrofuran provides very clean organic-water phase separations. It is a popular, but costlier substitute for tetrahydrofuran.
2-Methyltetrahydrofuran is approved by the United States Department of Energy as an additive to gasoline. Furfural and other partially hydrogenated/reduced furyl compounds between it and 2-methyltetrahydrofuran (furfuryl alcohol, methylfuran, tetrahydrofural alcohol) have a tendency to polymerize and are quite volatile. 2-Methyltetrahydrofuran itself, however, is more stable and less volatile, and thus is suitable for use as a motor fuel.
References
- ^ a b He, Man; Zhou, Da-Qing; Ge, Hong-Li; Huang, Mei-Yu; Jiang, Ying-Yan (2003). "Caytalytic Behavior of Wool-Rh Complex in Aymmetric Hydrogenation of 2-Methyl Furan". Polymer Advanced technology 14: 273–277. doi:10.1002/pat.305.
- ^ a b Aycock, David F. (2007). "Solvent Applications of 2-Methyltetrahydrofuran in Organometallic and Biphasic Reactions". Org. Proc. Res. Dev. 11: 156−159. doi:10.1021/op060155c.
- ^ Hoydonckx, H. E.; Van Rhijn, W. M.; Van Rhijn, W.; De Vos, D. E.; Jacobs, P. A. (2005), "Furfural and Derivatives", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a12_119.pub2
- Huber, George W.; Iborra, Sara; Corma, Avelino (2006). "Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering". Chemical Reviews 106: 4044–4098. doi:10.1021/cr068360d.
Further reading
- Zheng, Hong-Yan; Zhu, Yu-Lei; Teng, Bo-Tao; Bai, Zong-Qing; Zhang, Cheng-Hua; Xiang, Hong-Wei; Li, Yong-Wang (2006). "Towards understanding the reaction pathway in vapor phasehydrogenation of furfural to 2-methylfuran". Journal of Molecular Catalysis A, Chemical 246: 18. doi:10.1016/j.molcata.2005.10.003.
Categories:- Tetrahydrofurans
- Ether solvents
Wikimedia Foundation. 2010.