- Deuterated dichloromethane
-
Deuterated dichloromethane 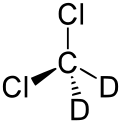
Identifiers CAS number 1665-00-5 PubChem 160586 ChemSpider 141111 
EC number 216-776-0 UN number 1593 Beilstein Reference 1733318 Jmol-3D images Image 1 - [2H]C([2H])(Cl)Cl
Properties Molecular formula C2H2Cl2 Molar mass 86.945 g mol-1 Exact mass 85.965908970 g mol-1 Density 1.362 g cm-3 Boiling point 40 °C, 313 K, 104 °F
Vapor pressure 52.6 kPa (at 20 °C) Hazards EU classification  Xn
XnR-phrases R40 S-phrases S23, S24/25, S36/37 NFPA 704 Related compounds Related compounds Deuterated chloroform
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Deuterated dichloromethane (CD2Cl2) is a form (called an isotopologue) of dichloromethane (DCM, CH2Cl2) in which the hydrogen atoms ("H") are replaced with deuterium (heavy hydrogen) isotope ("D"). Deuterated DCM is not a common solvent used in NMR spectroscopy as it is reasonably expensive.
Deuterated NMR Solvents Deuterated Deuterated acetone - (CD3)2C=O • Deuterated benzene - C6D6 • Deuterated chloroform - CDCl3 • Deuterated dichloromethane - CD2Cl2 • Deuterated DMF - (CD3)2NCOD • Deuterated DMSO - (CD3)2S=O • Deuterated ethanol - C2D5OD • Deuterated methanol - CD3OD • Deuterated THF - (C4D8)O • Deuterated water - D2O
Reference: 1H and 13C chemical shifts
This article about an organic compound is a stub. You can help Wikipedia by expanding it.

