- Deuterated DMSO
-
Deuterated DMSO 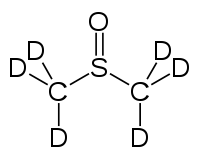 Trideuterio(trideuteriomethylsulfinyl)methaneSystematic name(2H3)Methanesulfinyl(2H3)methaneOther namesDeuterated dimethyl sulfoxide
Trideuterio(trideuteriomethylsulfinyl)methaneSystematic name(2H3)Methanesulfinyl(2H3)methaneOther namesDeuterated dimethyl sulfoxideIdentifiers Abbreviations DMSO-d6 CAS number 2206-27-1 
PubChem 75151 (anhydrate) 
ChemSpider 67699 (anhydrate) 
EC number 218-617-0 RTECS number PV6210000 Beilstein Reference 1237248 Jmol-3D images Image 1 - [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H]
Properties Molecular formula C2D6OS Molar mass 84.17 g/mol Exact mass 84.051596 u  DMSO (verify) (what is:
DMSO (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Deuterated DMSO, also knows as dimethyl sulfoxide-d6, is an isotopologue of dimethyl sulfoxide (DMSO, (CH3)2S=O)) with chemical formula ((CD3)2S=O) in which the hydrogen atoms ("H") are replaced with their isotope deuterium ("D"). Deuterated DMSO is a common solvent used in NMR spectroscopy.
Production
Deuterated DMSO is produced by heating DMSO in heavy water (D2O) with a basic catalyst such as calcium oxide. The reaction does not give complete conversion to the d6 product, and the water produced must be removed and replaced with D2O several times to drive the equilibrium to the fully deuterated product.[1]
Use in NMR Spectroscopy
Pure deuterated DMSO shows no peaks in 1H NMR spectroscopy so is commonly used as an NMR solvent. However commercially available samples are not 100% pure and a DMSO quintet peak will be observed in the regions 2.54ppm for 1H NMR and 40.45ppm in 13C NMR.[2]
References
Deuterated NMR Solvents Deuterated Deuterated acetone - (CD3)2C=O • Deuterated benzene - C6D6 • Deuterated chloroform - CDCl3 • Deuterated dichloromethane - CD2Cl2 • Deuterated DMF - (CD3)2NCOD • Deuterated DMSO - (CD3)2S=O • Deuterated ethanol - C2D5OD • Deuterated methanol - CD3OD • Deuterated THF - (C4D8)O • Deuterated water - D2O
Reference: 1H and 13C chemical shifts
This article about an organic compound is a stub. You can help Wikipedia by expanding it.
