- Danishefsky's diene
-
Danishefsky's diene[1]  Other namesKitahara diene
Other namesKitahara diene
trans-1-Methoxy-3-trimethylsilyloxy-1,3-butadiene
(E)-1-Methoxy-3-trimethylsilyloxy-1,3-butadieneIdentifiers CAS number 54125-02-9 PubChem 5366448 ChemSpider 4518295 
Jmol-3D images Image 1 - O(\C=C\C(O[Si](C)(C)C)=C)C
Properties Molecular formula C8H16O2Si Molar mass 172.3 g mol−1 Exact mass 172.091956289 g mol–1 Density 0.89 g cm–3 (20 °C)[2] Boiling point 68-69 °C, 341-342 K, 154-156 °F (at 0.0189 kPa[2])
Hazards R-phrases - S-phrases S23, S24/25  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Danishefsky’s diene (Kitahara diene) is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-1,3-butadiene named after Samuel J. Danishefsky.[3] Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The OMe group promotes highly regioselective additions.
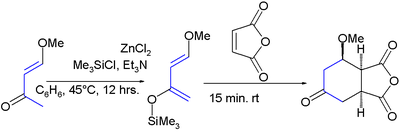
It was first synthesized by the reaction of trimethylsilyl chloride with 4-methoxy-3-buten-2-one and zinc chloride:[4]
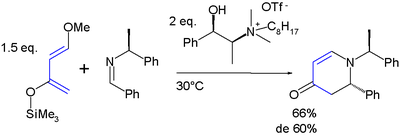
The diene has two features of interest: the substituents promote regiospecific addition to unsymmetrical dienophiles and the resulting adduct is amenable to further functional group manipulations after the addition reaction. High regioselectivity is obtained with unsymmetrical alkenes with a preference for an 1,2-relation of the ether group with the electron-deficient alkene-carbon. All this is exemplified in this Aza Diels-Alder reaction:[5][6]
In the cycloaddition product, the silyl ether is a synthon for a carbonyl group via the enol. The methoxy group is susceptible to an elimination reaction enabling the formation of a new alkene group.
References
- ^ Sicherheitsdatenblatt Sigma-Aldrich.
- ^ a b Sicherheitsdatenblatt Merck.
- ^ Samuel J. Danishefsky; Kitahara, T. Useful diene for the Diels-Alder reaction. J. Am. Chem. Soc. 1974, 96, 7807-7808. doi:10.1021/ja00832a031
- ^ Preparation and Diels-Alder Reaction of a Highly Nuclerophilic Diene. Org. Synth., Coll. Vol. 7, p.312 (1990); Vol. 61, p.147 (1983). Link
- ^ Asymmetric aza-Diels-Alder reaction of Danishefsky's diene with imines in a chiral reaction medium Pegot B, Nguyen Van Buu O, Gori D, Vo-Thanh G Beilstein Journal of Organic Chemistry, 2006 Link
- ^ This is an asymmetric reaction with a chiral ionic liquid as chiral solvent. The reported chemical yield is 66% with 60% diastereomeric excess
Wikimedia Foundation. 2010.