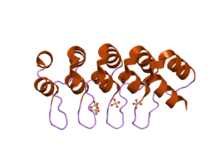- DARPin
-
DARPins (an acronym for designed ankyrin repeat proteins) are genetically engineered antibody mimetic proteins typically exhibiting highly specific and high-affinity target protein binding. They are derived from natural ankyrin proteins and consist of at least three, usually four or five repeat motifs of these proteins. Their molecular mass is about 14 or 18 kDa (kilodaltons) for four- or five-repeat DARPins, respectively.
DARPins are used as investigational tools, and diagnostic and therapeutic applications are being aimed at. In 2010, the first of these molecules, codenamed MP0112, entered clinical trials[1].
Contents
Origin, structure and generation
DARPins have been developed mainly at the University of Zurich, Switzerland[2]. They are derived from naturally occurring ankyrin proteins, a protein class that is mediating high-affinity protein-protein interactions in nature. Sequence alignments of several thousand natural ankyrin repeat motifs (of about 33 amino acids each) combined with structure based design and recombinant DNA methods is used for generation of these proteins[2]. DARPins are composed of repetitive structural units forming a stable protein domain with a large potential target interaction surface. Typically, DARPins are composed of four or five repeats, corresponding to the average size of natural ankyrin repeat protein domains. Proteins with less than three repeats do not form a tertiary structure.[3] The molecular mass depending on the number of repeats is as follows:
Repeats 3 4 5 6 7 ... Approximate mass (kDa) 10 14 18 22 26 ... Libraries of DARPins with randomized potential target interaction residues with diversities of over 1012 variants have been generated at the DNA level. From these libraries, DARPins binding the target of choice with picomolar affinity and specificity can be selected using ribosome display[4] or signal recognition particle (SRP) phage display[5]. DARPins can be designed to act as receptor agonists, antagonists, inverse agonists, enzyme inhibitors, or simple target protein binders[6].
Properties
DARPins are expressed in the cytoplasm of Escherichia coli at high levels (over 10 g/l in fermentation, 1 g/l in shake flask) in soluble form. The proteins exhibit high thermal and thermodynamic stability (denaturation midpoint: Tm > 66 °C, equilibrium unfolding: ∆G > 9.5 kcal/mol), which is increasing with increasing repeat number[2][7][8]. DARPins are stable in human blood serum and do not contain T-cell epitopes. The high specificity and affinity of binding DARPins has been attributed rigid body binding mode[4]. Multi-specific or multi-valent constructs made by genetic fusion show similar properties as single domain DARPins. The absence of cysteines in the scaffold enables engineering of site-specific cysteines, allowing site-directed coupling of chemicals to the molecule.
Applications and clinical development
DARPins have been used as research tools[9], as diagnostic agents[10] and as therapeutic agents[6]. Zurich based Molecular Partners AG is currently pursuing the development of therapeutic DARPins. MP0112, the first therapeutic DARPin candidate, is a vascular endothelial growth factor (VEGF) inhibitor and has entered clinical trials for the treatment of wet macular degeneration[11] and diabetic macular edema[1] in early 2010[12].
References
- ^ a b ClinicalTrials.gov NCT01042678 Study of MP0112 Intravitreal Injection in Patients With Diabetic Macula Edema
- ^ a b c Binz HK, Stumpp MT, Forrer P, Amstutz P, Plückthun A (September 2003). "Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins". Journal of Molecular Biology 332 (2): 489–503. doi:10.1016/S0022-2836(03)00896-9. PMID 12948497. http://linkinghub.elsevier.com/retrieve/pii/S0022283603008969.
- ^ Mosavi, L. K.; Minor, D. L.; Peng, Z. -Y. (2002). "Consensus-derived structural determinants of the ankyrin repeat motif". Proceedings of the National Academy of Sciences 99 (25): 16029–34. Bibcode 2002PNAS...9916029M. doi:10.1073/pnas.252537899. PMC 138559. PMID 12461176. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=138559.
- ^ a b Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P, Grütter MG, Plückthun A (May 2004). "High-affinity binders selected from designed ankyrin repeat protein libraries". Nature Biotechnology 22 (5): 575–582. doi:10.1038/nbt962. PMID 15097997. http://www.nature.com/nbt/journal/v22/n5/abs/nbt962.html.
- ^ Steiner D, Forrer P, Stumpp MT, Plückthun A (May 2006). "Signal sequences directing cotranslational translocation expand the range of proteins amenable to phage display". Nature Biotechnology 24 (7): 823–831. doi:10.1038/nbt1218. PMID 16823375. http://www.nature.com/nbt/journal/v24/n7/abs/nbt1218.html.
- ^ a b Stumpp MT, Binz HK, Amstutz P (August 2008). "DARPins: a new generation of protein therapeutics". Drug Discov. Today 13 (15–16): 695–701. doi:10.1016/j.drudis.2008.04.013. PMID 18621567. http://linkinghub.elsevier.com/retrieve/pii/S1359-6446(08)00162-1.
- ^ Kohl A, Binz HK, Forrer P, Stumpp MT, Plückthun A, Grütter MG (May 2003). "Designed to be stable: Crystal structure of a consensus ankyrin repeat protein". Proc. Natl Acad. Sci. USA 100 (4): 1700–1775. doi:10.1073/pnas.0337680100. PMC 149896. PMID 12566564. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=149896.
- ^ Wetzel SK, Settanni G, Kenig M, Binz HK, Plückthun A (February 2008). "Folding and unfolding mechanism of highly stable full-consensus ankyrin repeat proteins". Journal of Molecular Biology 376 (1): 241–257. doi:10.1016/j.jmb.2007.11.046. PMID 18164721. http://linkinghub.elsevier.com/retrieve/pii/S0022-2836(07)01530-6.
- ^ Bandeiras TM, Hillig RC, Matias PM, Eberspaecher U, Fanghänel J, Thomaz M, Miranda S, Crusius K, Pütter V, Amstutz P, Gulotti-Georgieva M, Binz HK, Holz C, Schmitz AA, Lang C, Donner P, Egner U, Carrondo MA, Müller-Tiemann B (April 2008). "Structure of wild-type Plk-1 kinase domain in complex with a selective DARPin". Acta Crystallogr D D64 (4): 339–353. doi:10.1107/S0907444907068217. PMID 18391401. http://scripts.iucr.org/cgi-bin/paper?S0907444907068217.
- ^ Theurillat JP, Dreier B, Nagy-Davidescu G, Seifert B, Behnke S, Zürrer-Härdi U, Ingold F, Plückthun A, Moch H (2010). "Designed ankyrin repeat proteins: a novel tool for testing epidermal growth factor receptor 2 expression in breast cancer". Mod Pathol. 23 (9): 1289–97. doi:10.1038/modpathol.2010.103. PMID 20495541. http://www.nature.com/modpathol/journal/vaop/ncurrent/abs/modpathol2010103a.html.
- ^ ClinicalTrials.gov NCT01086761 Study of MP0112 Intravitreal Injection in Patients With Wet Age Related Macular Degeneration
- ^ Heinzelmann, E (December 2008). "DARPins – Allrounder für Diagnose und Therapie" (in German). Swiss Engineering: 62–63. http://www.swissengineering-stz.ch/pdf/stz1220083130.pdf.
Engineered monoclonal antibodies and antibody mimetics Whole antibody bispecific: Trifunctional antibody
Fab fragment Variable fragment Single-chain variable fragment / di-scFv / tri-scFv • Single-domain antibody • Small modular immunopharmaceutical • bispecific: T-cell engagerSmaller units Intracellular IntrabodyAntibody mimetics Categories:- Molecular biology
- Proteins
Wikimedia Foundation. 2010.