- Coelenterazine
-
Coelenterazine 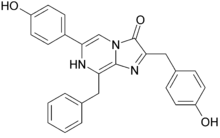 6-(4-hydroxyphenyl)-2-[(4-hydroxyphenyl)methyl]-8-(phenylmethyl)-7H-imidazo[3,2-a]pyrazin-3-oneOther namesCoelenterazine, Renilla luciferin
6-(4-hydroxyphenyl)-2-[(4-hydroxyphenyl)methyl]-8-(phenylmethyl)-7H-imidazo[3,2-a]pyrazin-3-oneOther namesCoelenterazine, Renilla luciferinIdentifiers CAS number 55779-48-1 
PubChem 2830 ChemSpider 2728 
ChEBI CHEBI:2311 
Jmol-3D images Image 1 - C1=CC=C(C=C1)CC2=C3N=C(C(=O)N3C=C(N2)C4=CC=C(C=C4)O)CC5=CC=C(C=C5)O
- InChI=1S/C26H21N3O3/c30-20-10-6-18(7-11-20)15-23-26(32)29-16-24(19-8-12-21(31)13-9-19)27-22(25(29)28-23)14-17-4-2-1-3-5-17/h1-13,16,27,30-31H,14-15H2

Key: YHIPILPTUVMWQT-UHFFFAOYSA-N
InChI=InChI=1S/C26H21N3O3/c30-20-10-6-18(7-11-20)15-23-26(32)29-16-24(19-8-12-21(31)13-9-19)27-22(25(29)28-23)14-17-4-2-1-3-5-17/h1-13,16,27,30-31H,14-15H2
Properties Molecular formula C26H21N3O3 Molar mass 423.463 Appearance orange-yellow crystals Melting point 175-178°C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Coelenterazine is the luciferin, the light-emitting molecule, found in many aquatic organisms across seven phyla.[1] It is the substrate in many luciferases and photoproteins including Renilla reniformis luciferase (Rluc), Gaussia luciferase (Gluc), aequorin, and obelin.
Contents
History
Coelenterazine was simultaneously isolated and characterized by two groups studying the luminescent organisms sea pansy (Renilla reniformis) and the coelenterate Aequorea victoria, respectively.[2][3] Both groups unknowingly discovered that the same compound was used in both luminescent systems, however the name of the molecule was given after the coelenterate. Likewise, the two main metabolites - coelenteramide and coelenteramine - were named after their respective functional groups.
Occurrence
Coelenterazine is widely found in marine organisms including:
- radiolarians
- ctenophores
- cnidarians such as Aequorea victoria, Obelia geniculata and Renilla reniformis
- squid such as Watasenia scintillans and Vampyroteuthis infernalis
- shrimp such as Systellaspis debilis and Oplophorus gracilirostris
- copepods such as Pleuromamma xiphias and Gaussia princeps
- chaetognaths[4]
- fish including some Neoscopelidae and Myctophidae
- echinoderms such as Amphiura filiformis
The compound has also been isolated from organisms that are not luminescent, such as the Atlantic herring and several shrimp species including Pandalus borealis and Pandalus platyuros.
Properties
Coelenterazine can be crystallized into orange-yellow crystals. The molecule absorbs light in the ultraviolet and visible spectrum, with peak absorption at 435 nm in methanol, giving the molecule a yellow color. The molecule spontaneously oxidizes in aerobic conditions or in some organic solvents such as dimethylformamide and DMSO and is preferentially stored in methanol or with an inert gas.
References
- ^ Shimomura, O. (2006). Bioluminescence: Chemical Principles and Methods. World Scientific Publishing. ISBN 9789812568014.
- ^ Hori K, Charbonneau H, Hart RC, and Cormier MJ (1977). "Structure of native Renilla reinformis luciferin". Proceedings of the National Academy of Sciences 74 (10): 4285–4287. doi:10.1073/pnas.74.10.4285. PMC 431924. PMID 16592444. http://www.pnas.org/content/74/10/4285.abstract.
- ^ Shimomura O, Johnson FH (1975). "Chemical nature of bioluminescence systems in coelenterates". Proceedings of the National Academy of Sciences 72 (4): 1546–1549. doi:10.1073/pnas.72.4.1546. PMC 432574. PMID 236561. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=432574.
- ^ Haddock SHD, Case JF (1994). "A bioluminescent chaetognath". Nature 367 (6460): 225–226. doi:10.1038/367225a0. http://www.lifesci.ucsb.edu/~haddock/abstracts/haddock_chaeto.pdf.
External links
- Bioluminescence Page showing major luciferin types
Categories:- Bioluminescence
- Aminopyrazines
- Imidazopyrazines
- Lactams
Wikimedia Foundation. 2010.
