- Coelenteramide
-
Coelenteramide 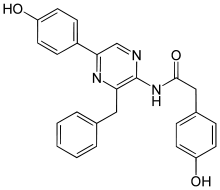 N-[2-benzyl-6-(4-oxocyclohexa-2,5-dien-1-ylidene)-1H-pyrazin-3-yl]-2-(4-hydroxyphenyl)acetamideOther namesCoelenteramide
N-[2-benzyl-6-(4-oxocyclohexa-2,5-dien-1-ylidene)-1H-pyrazin-3-yl]-2-(4-hydroxyphenyl)acetamideOther namesCoelenteramideIdentifiers PubChem 5326781 Jmol-3D images Image 1 - C1=CC=C(C=C1)CC2=C(N=CC(=C3C=CC(=O)C=C3)N2)NC(=O)CC4=CC=C(C=C4)O
Properties Molecular formula C25H21N3O3 Molar mass 411.45254  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Coelenteramide is the oxidized product, or oxyluciferin, of the bioluminescent reactions in many marine organisms that utilize coelenterazine. It was first isolated as a blue fluorescent compound from Aequorea victoria after the animals were stimulated to emit light.[1] Under basic conditions, the compound will break down further into coelenteramine and 4-hydroxyphenylacetic acid.
It is an aminopyrazine.[2]
References
- ^ Shimomura O, Johnson FH (1975). "Chemical Nature of Bioluminescence Systems in Coelenterates". Pnas USA 72 (4): 1546–1549. doi:10.1073/pnas.72.4.1546. PMC 432574. PMID 236561. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=432574.
- ^ Discovery and Validation of a New Family of Antioxidants: The Aminopyrazine Derivatives. M. L. N. Dubuisson, J.-F. Rees and J. Marchand-Brynaert, Mini-Reviews in Medicinal Chemistry, 2004, 4, 159-165, doi:10.2174/1389557043403927
This article about a heterocyclic compound is a stub. You can help Wikipedia by expanding it.
