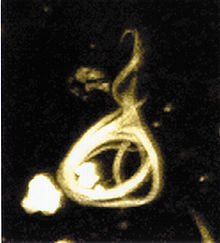
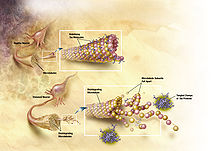
- Neurofibrillary tangle
-
Neurofibrillary Tangles (NFTs) are aggregates of hyperphosphorylated tau protein that are most commonly known as a primary marker of Alzheimer's Disease. Their presence is also found in numerous other diseases known as Tauopathies. Little is known about their exact relationship to the different pathologies.
Contents
Formation
Neurofibrillary tangles are formed by hyperphosphorylation of a microtubule-associated protein known as tau, causing it to aggregate, or group, in an insoluble form. (These aggregations of hyperphosphorylated tau protein are also referred to as PHF, or "paired helical filaments"). The precise mechanism of tangle formation is not completely understood, and it is still controversial whether tangles are a primary causative factor in disease or play a more peripheral role.
Cytoskeletal changes
Three different maturation states of NFT have been defined using anti-tau and anti-ubiquitin immunostaining. At stage 0 there are morphologically normal pyramidal cells showing diffuse or fine granular cytoplasmic staining with anti-tau. In other words cells are healthy with minimal tau presence; at stage 1 some delicate elongate inclusions are stained by tau antibodies (these are early tangles); stage 2 is represented by the classic NFT demonstration with anti-tau staining ; stage 3 is exemplified by ghost tangles (tangles outside of cells where the host neuron has died), which are characterized by a reduced anti-tau but marked anti-ubiquitin immunostaining.[1]
Causes
Mutated Tau
The traditional understanding is that tau binds to microtubules and assists with their formation and stabilization. However when tau is hyperphosphorylated, it is unable to bind and the microtubules become unstable and begin disintegrating. The unbound tau clumps together in formations called neurofibrillary tangles.[2] More explicitly, intracellular lesions known as pretangles develop when tau is phosphorylated excessively and on improper amino acid residues. These lesions, over time, develop into filamentous neurofibrilary tangles (NFTs) which interfere with numerous intracellular functions. Seeking a reliable animal model for tau-related pathologies, researchers expressed the human mutant P301L tau gene in adult mice. This experiment resulted in the formation of neurofibrillary tangles and pretangle formations. The human mutant P301 tau gene is associated with frontotemporal dementia with parkinsonism, another tauopathy associated with NFTs. It was found that the degree of tau pathology was dependent on time and the level of gene expression.[3] Groups receiving a combination of a promoter and enhancer in the vector saw increased tau expression, as early as 3 weeks after vector injection, which was measured using a Western blot.[3] These groups also showed a greater pathology compared to those with less expression of the mutant tau. Additionally, NFTs were clearly detected by immunoelectron microscopy at 4 months but not at 2 months. However, at both 2 and 4 months, pretangle-like structures were observed suggesting the NFT formation is not complete by 4 months and will continue to progress with time.[3]
Traumatic Brain Injury
Preliminary research indicates that iron deposits due to hemorrhaging, following traumatic brain injury (TBI), may increase tau pathology. While TBI does not routinely lead to accelerated NFT formation, further work may determine if other blood components or factors unrelated to hemorrhages are involved in this TBI-induced augmentation of tau pathology.[4] NFTs are most commonly seen associated with repetitive mild TBI as opposed to one instance of severe traumatic brain injury.[5] For example the clinical syndrome of dementia pugilistica, otherwise known as punch-drunk syndrome found in boxers, is highly associated with NFTs and neuropil threads.
Aluminum
The idea that there is a link between aluminum exposure and the formation of neurofibrillary tangles has floated around the scientific community for sometime without having been definitively proven or disregarded. Recently a study examining the hippocampal CA1 cells from individuals with and with out Alzheimer’s disease showed a small portion of the pyramidal cells contain cytoplasmic pools within their somas containing early NFTs. These cytoplasmic pools are aggregates of an aluminum/hyperphosphorylated tau complex similar to mature NFTs. (Walton) While a connection between aluminum and NFTs and AD is maintained, there is evidence that aluminum does not directly cause the formation of NFTs or AD.[6]
Pathology
It has been shown that the degree of cognitive impairment in diseases such as AD is significantly correlated with the presence of neurofibrillary tangles.[7]
Harmful or Protective?
There has been some suggestion that the formation of NFTs is not a causal relationship with disease. Rather that NFTs may be produced in response to a variety of conditions and may in fact be a compensatory response against oxidative stress and serves a protective function. Several points are made to argue the position that NFTs are perhaps protective instead of harmful. First there appears to be a dispute as to the impact of neurofibrillary tangles on neuronal viability because some neurons containing NFTs survive for decades.[2] Furthermore, NFTs have been found in apparently healthy individuals, indicating that NFTs are not directly related to neural degeneration. It has been proposed that the formation of NFTs is part of a multifaced compensatory response where oxidative insult activates several kinases, which are then capable of phosphorylating tau. This then prompts the early formation of NFTs, which reduce oxidative damage and prolong the function of the neuron.[2] While an intriguing theory, scientists have not come to a firm conclusion as to what role NFTs play in neurodegenerative diseases.
Neuron loss
Traditionally believed to play a major role in neuron loss, NFTs are an early event in pathologies such as Alzheimer’s disease, and as more NFTs form, there is substantially more neuron loss. However, it has been shown that there is significant neuron loss before the formation of neurofibrillary tangles, and that NFTs account for only a small proportion (around 8.1%) of this neuron loss.[8] Coupled with the longevity of neurons containing NFTs, it is likely that some other factor is primarily responsible for the bulk of neuron loss in these diseases, not the formation of neurofibrillary tangles.
NFT-predominant dementia vs. Classical Alzheimer’s
It is currently unclear as to whether or not Neurofibrillary tangle-predominant dementia (NFTPD) (a.k.a. tangle-only dementia) is a variant of the traditional Alzheimer’s disease, or a genetically distinct entity. Characterized by later onset and milder cognitive impairment, the distribution of NFT pathology is more closely related to that found in centenarians showing no or limited cognitive impairment. NFTs are generally limited to allocortical/limbic regions of the brain with limited progression to the neocortex but a greater density in the allocortical/hippocampal region. Plaques are generally absent.[9]
Alzheimer disease with concomitant dementia with Lewy bodies (AD+DLB)
The degree of NFT involvement in AD is defined by Braak stages. Braak stages I and II are used when NFT involvent is confined mainly to the transentorhinal region of the brain. Stages III and IV indicated involvement of limbic regions such as the hippocampus, and V and VI when there's extensive neocortical involvement. This should not be confused with the degree of senile plaque involvement, which progresses differently.[10]
Neurofibrillary tangle and modified Braak scores were lower in AD+DLB, however, neocortical NFT scores show markedly different patterns between AD+DLB and Classical Alzheimers. In pure AD, NFT are predominately found at a high frequency: In AD+DLB, the distribution of NFT frequency was found to be bimodal: NFTs were either frequent or few to absent. Additionally, neocortical NFT frequency in the AD+DLB group tended to parallel the severity of other types of tau cytopathology.[11]
Link to Aggression and Depression In Alzheimer's Patients
A recent study looked for correlation between the quantitative aspects of Alzheimer's disease (neuron loss, neuritic plaque and neurofibrillary tangle load) and aggression frequently found in Alzheimer's patients. It was found that only an increase in neurofibrillary tangle load was associated with severity of aggression and chronic aggression in Alzheimer's patients.[12] While this study does indicate a correlation between NFT load and severity of aggression, it does not provide a causative argument.
Research has also indicated that patients with AD and comorbid depression show higher levels of neurofibrillary tangle formation than individuals with AD but no depression.[13] Comorbid depression increased the odds for advanced neuropathologic disease stage even when controlling for age, gender, education and cognitive function.[13]
Treatment
Statins
Statins have been shown to reduce the neurofibrillary tangle burden in mouse models, likely due to their anti-inflammatory capacities.[14]
Cyclin-dependent kinase 5
Cyclin-dependent kinase 5 (CDK5) is a kinase that has been previously hypothesized to contribute to tau pathologies. RNA interference (RNAi) mediated silencing of the CDK5 gene has been proposed as a novel therapeutic strategy again tau pathology, such as neurofibrillary tangles. Knockdown of CDK5 has been shown to reduce the phosphorylation of tau in primary neuronal cultures and in mouse models. Furthermore, this silencing showed a dramatic reduction in the number of neurofibrillary tangles.[15]
Lithium
Lithium has been shown to decrease the phosphorylation of tau.[16] Lithium treatment has been shown to reduce the density of neurofibrillary tangles in transgenic models in the hippocampus and spinal cord. Despite the decrease in density of NFTs, motor and memory deficits were not seen to improve following treatment. Additionally, no preventative effects have been seen in patients undergoing lithium treatment.[16]
Other conditions
- Progressive supranuclear palsy[17] although with straight filament rather than PHF tau
- Dementia pugilistica (chronic traumatic encephalopathy)[18]
- Frontotemporal dementia and parkinsonism linked to chromosome 17 however without detectable β-amyloid plaques.[19]
- Lytico-Bodig disease (Parkinson-dementia complex of Guam)[20]
- Ganglioglioma and gangliocytoma[21]
- Meningioangiomatosis[22]
- Subacute sclerosing panencephalitis[23]
- As well as lead encephalopathy, tuberous sclerosis, Hallervorden-Spatz disease, and lipofuscinosis[24]
See also
References
- ^ Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Wisniewski HM et al. (1989). "Accumulation of abnormally phosphorylated x precedes the formation of neurofibrillary tangles in Alzheimer's disease". Brain Res 477 (1–2): 90–99. doi:10.1016/0006-8993(89)91396-6. PMID 2495152.
- ^ a b c Lee H. G., Perry G., Moreira P. I., Garrett M. R., Liu Q., Zhu X. W. et al. (2005). "Tau phosphorylation in Alzheimer's disease: pathogen or protector?". Trends in Molecular Medicine 11 (4): 164–169. doi:10.1016/j.molmed.2005.02.008. PMID 15823754.
- ^ a b c Klein R. L., Lin W. L., Dickson D. W., Lewis J., Hutton M., Duff K. et al. (2004). "Rapid neurofibrillary tangle formation after localized gene transfer of mutated tau". American Journal of Pathology 164 (1): 347–353. doi:10.1016/S0002-9440(10)63124-0. PMC 1602230. PMID 14695347. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1602230.
- ^ Yoshiyama Y., Uryu K., Higuchi M., Longhi L., Hoover R., Fujimoto S. et al. (2005). "Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. [Article]". Journal of Neurotrauma 22 (10): 1134–1141. doi:10.1089/neu.2005.22.1134. PMID 16238489.
- ^ DeKosky S. T., Ikonomovic M. D., Gandy S. (2010). "Traumatic Brain Injury -- Football, Warfare, and Long-Term Effects". New England Journal of Medicine 363 (14): 1293–1296. doi:10.1056/NEJMp1007051. PMID 20879875.
- ^ Edwardson JA, Candy JM, Ince PG, et al. (1992). "Aluminium accumulation, beta-amyloid deposition and neurofibrillary changes in the central nervous system". Ciba Found. Symp. 169: 165–79. doi:10.1002/9780470514306.ch10. PMID 1490421.
- ^ Braskie M. N., Klunder A. D., Hayashi K. M., Protas H., Kepe V., Miller K. J. et al. (2010). "Plaque and tangle imaging and cognition in normal aging and Alzheimer's disease. [Article"]. Neurobiology of Aging 31 (10): 1669–1678. doi:10.1016/j.neurobiolaging.2008.09.012. PMC 2891885. PMID 19004525. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2891885.
- ^ Kril J. J., Patel S., Harding A. J., Halliday G. M. (2002). "Neuron loss from the hippocampus of Alzheimer's disease exceeds extracellular neurofibrillary tangle formation. [Article]". Acta Neuropathologica 103 (4): 370–376. doi:10.1007/s00401-001-0477-5. PMID 11904757.
- ^ Jellinger, K. A.; Attems, J. (2006). "Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease". Acta Neuropathologica 113 (2): 107–17. doi:10.1007/s00401-006-0156-7. PMID 17089134.
- ^ Braak, H.; Braak, E. (1991). "Neuropathological stageing of Alzheimer-related changes". Acta Neuropathologica 82 (4): 239–59. doi:10.1007/BF00308809. PMID 1759558.
- ^ Gearing, M., Lynn, M., & Mirra, S. S. (Feb 1999). "Neurofibrillary pathology in Alzheimer disease with Lewy bodies - Two subgroups". Archives of Neurology 56 (2): 203–208. doi:10.1001/archneur.56.2.203. PMID 10025425.
- ^ Lai M. K. P., Chen C. P., Hope T., Esiri M. M. (2010). "Hippocampal neurofibrillary tangle changes and aggressive behaviour in dementia". Neuroreport 21 (17): 1111–1115. doi:10.1097/WNR.0b013e3283407204. PMID 20890229.
- ^ a b Rapp M. A., Schnaider-Beeri M., Purohit D. P., Perl D. P., Haroutunian V., Sano M. (2008). "Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression". American Journal of Geriatric Psychiatry 16 (2): 168–174. doi:10.1097/JGP.0b013e31816029ec. PMID 18239198.
- ^ Boimel M., Grigoriadis N., Lourbopoulos A., Touloumi O., Rosenmann D., Abramsky O. et al. (2009). "Statins Reduce the Neurofibrillary Tangle Burden in a Mouse Model of Tauopathy. [Article]". Journal of Neuropathology and Experimental Neurology 68 (3): 314–325. doi:10.1097/NEN.0b013e31819ac3cb. PMID 19225406.
- ^ Piedrahita D., Hernandez I., Lopez-Tobon A., Fedorov D., Obara B., Manjunath B. S. et al. (2010). "Silencing of CDK5 Reduces Neurofibrillary Tangles in Transgenic Alzheimer's Mice". Journal of Neuroscience 30 (42): 13966–13976. doi:10.1523/JNEUROSCI.3637-10.2010. PMC 3003593. PMID 20962218. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3003593.
- ^ a b Leroy K., Ando K., Heraud C., Yilmaz Z., Authelet M., Boeynaems J. M. et al. (2010). "Lithium Treatment Arrests the Development of Neurofibrillary Tangles in Mutant Tau Transgenic Mice with Advanced Neurofibrillary Pathology" (PDF). Journal of Alzheimers Disease 19 (2): 705–719. PMID 20110614. http://www.j-alz.com/issues/19/leroy_supplement.pdf.
- ^ Williams, David R; Lees, Andrew J (2009). "Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges". The Lancet Neurology 8 (3): 270–9. doi:10.1016/S1474-4422(09)70042-0.
- ^ Roberts, GW (1988). "Immunocytochemistry of neurofibrillary tangles in dementia pugilistica and Alzheimer's disease: evidence for common genesis". Lancet 2 (8626–8627): 1456–8. doi:10.1016/S0140-6736(88)90934-8. PMID 2904573. http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(88)90934-8.
- ^ Selkoe, Dennis J.; Podlisny, Marcia B. (2002). "Deciphering the genetic basis of Alzheimer's disease". Annual Review of Genomics and Human Genetics 3: 67–99. doi:10.1146/annurev.genom.3.022502.103022. PMID 12142353.
- ^ Hof PR, Nimchinsky EA, Buée-Scherrer V, et al. (1994). "Amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam: quantitative neuropathology, immunohistochemical analysis of neuronal vulnerability, and comparison with related neurodegenerative disorders". Acta Neuropathol. 88 (5): 397–404. doi:10.1007/BF00389490. PMID 7847067.
- ^ Brat, Daniel J.; Gearing, Marla; Goldthwaite, Patricia T.; Wainer, Bruce H.; Burger, Peter C. (2001). "Tau-associated neuropathology in ganglion cell tumours increases with patient age but appears unrelated to ApoE genotype". Neuropathology and Applied Neurobiology 27 (3): 197–205. doi:10.1046/j.1365-2990.2001.00311.x. PMID 11489139.
- ^ Halper, J; Scheithauer, BW; Okazaki, H; Laws Jr, ER (1986). "Meningio-angiomatosis: a report of six cases with special reference to the occurrence of neurofibrillary tangles". Journal of neuropathology and experimental neurology 45 (4): 426–46. PMID 3088216.
- ^ Paula-Barbosa, M. M.; Brito, R.; Silva, C. A.; Faria, R.; Cruz, C. (1979). "Neurofibrillary changes in the cerebral cortex of a patient with subacute sclerosing panencephalitis (SSPE)". Acta Neuropathologica 48 (2): 157–60. doi:10.1007/BF00691159. PMID 506699.
- ^ Wisniewski, Krystyna; Jervis, George A.; Moretz, Roger C.; Wisniewski, Henryk M. (1979). "Alzheimer neurofibrillary tangles in diseases other than senile and presenile dementia". Annals of Neurology 5 (3): 288–94. doi:10.1002/ana.410050311. PMID 156000.
External links
- Pathologic page about Neurofibrillary tangles, by the University of Oklahoma.
- http://www.termedia.pl/magazine.php?magazine_id=20&article_id=5368&magazine_subpage=ABSTRACT
- http://www.lifesci.sussex.ac.uk/home/Julian_Thorpe/ad_cyto.htm#tau
- It Takes Tau to Tangle : Plaques, Tangles and Neurodegenerative Disease (requires Flash video software)
- The Truth about Neurofibrillary Tangles (Flash video)
- Neurofibrillary Tangles - a definition (Flash video)
- Neurofibrillary Tangles in Alzheimer's Disease (Flash video)
Categories:- Dementia
- Medical signs
- Neurology
- Histopathology
Wikimedia Foundation. 2010.