- Trigonelline
-
Trigonelline 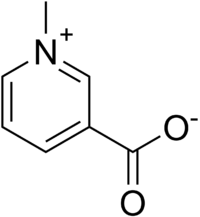 1-Methylpyridinium- 3-carboxylateOther namesNicotinic acid N- methylbetaine
1-Methylpyridinium- 3-carboxylateOther namesNicotinic acid N- methylbetaine
Coffearine
Caffearine
Gynesine
TrigenollineIdentifiers CAS number 535-83-1 
PubChem 5570 ChemSpider 5369 
ChEBI CHEBI:18123 
ChEMBL CHEMBL350675  , CHEMBL489961
, CHEMBL489961Jmol-3D images Image 1 - O=C([O-])c1ccc[n+](c1)C
Properties Molecular formula C7H7NO2 Molar mass 137.14 g mol−1 Density ? g/cm3 Melting point 230-233 °C (monohydrate)
258-259 °C (hydrochloride) (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Trigonelline is an alkaloid with chemical formula C7H7NO2. It is an inner salt formed by the addition of a methyl group to the nitrogen atom of niacin. Trigonelline is a product of the metabolism of niacin (vitamin B3) which is excreted in the urine.[1]
Trigonelline occurs in many plants, it was isolated from fenugreek seeds (Trigonella foenum-graecum, thus the name),[2] garden peas, hemp seed, oats,[3] potatoes, Stachys species, dahlia,[4] Strophanthus species[5] and Dichapetalum cymosum.[6] Holtz, Kutscher and Theilmann have recorded its presence in a number of animals.[7]
Trigonelline is also found in coffee,[8] where it may help to prevent dental caries by preventing the bacteria Streptococcus mutans from adhering to teeth.[9] Higher levels of trigonelline is found in robusta coffee.
Trigonelline crystallises as a monohydrate from alcohol in hygroscopic prisms, mp. 130 °C or 218 °C (dry, dec.). It is readily soluble in water or warm alcohol, less so in cold alcohol, and slightly so in chloroform or ether. The salts crystallise well, the monohydrochloride, in leaflets, sparingly soluble in dry alcohol. The picrate forms shining prisms, mp. 198-200 °C, soluble in water but sparingly soluble in dry alcohol or ether. The alkaloid forms several aurichlorides: the normal salt, B•HCl•AuCl3, is precipitated when excess of gold chloride is added to the hydrochloride, and after crystallisation from dilute hydrochloric acid containing some gold chloride, has mp. 198 °C. Crystallised from water or very dilute hydrochloric acid, slender needles of B4•3 HAuCl4, mp. 186 °C, are obtained.
References
- ^ Merck Index, 11th Edition, 9606.
- ^ Jahns, Ber., 1885, 18, 2518.
- ^ Schulze and Frankfurt, Ber., 1894, 27, 709.
- ^ Schulze and Trier, Zeit. physiol. Chem., 1912, 76, 258.
- ^ Thoms, Ber., 1891, 31, 271, 404.
- ^ Rimington, Onderstepoort J., 1935, 5, 81.
- ^ Zeit. Biol., 1924, 81, 57.
- ^ Gorter, Annalen, 1910, 372, 237; cf. Polstorff, Chem. Soc. Abstr., 1910, ii, 234; Palladino, ibid, 1894, ii, 214; 1895, i, 629; Graf, ibid, 1904, i, 915; Nottbohm and Mayer, Zeit. Unters. Lebensmitt., 1931, 61, 429.
- ^ Daglia, M.; R. Tarsi, A. Papetti, P. Grisoli, C. Dacarro, C. Pruzzo, and G. Gazzani (2002). "Antiadhesive Effect of Green and Roasted Coffee on Streptococcus mutans' Adhesive Properties on Saliva-Coated Hydroxyapatite Beads". Journal of Agricultural and Food Chemistry 50 (5): 1225–1229. doi:10.1021/jf010958t. PMID 11853508.
Categories:- Alkaloids
- Coffee chemistry
- Quaternary ammonium compounds
- Nicotinates
Wikimedia Foundation. 2010.
