- Trimethyltin chloride
-
Trimethyltin chloride 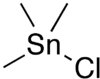 Other nameschlorotrimethylstannane; chlorotrimethyltin; trimethyl chlorostannane; trimethylchlorotin; trimethylstannyl chloride; trimethyltin monochloride
Other nameschlorotrimethylstannane; chlorotrimethyltin; trimethyl chlorostannane; trimethylchlorotin; trimethylstannyl chloride; trimethyltin monochlorideIdentifiers CAS number 1066-45-1 Properties Molecular formula C3H9SnCl Molar mass 199.27 g/mol Melting point 38.5 °C (311.65 K)[1]
Boiling point 148 °C (421.15 K)
Hazards MSDS External MSDS R-phrases 26/27/28-50/53 S-phrases 26-27-28-45-60-61  chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Trimethyltin chloride is an organotin compound]] with the formula (CH3)3SnCl. It is a white solid that is highly toxic and has a stench. It is susceptible to hydrolysis.
Synthesis
Trimethyltin chloride can be prepared by the redistribution reaction of tetramethyltin with tin tetrachloride.[2]
- SnCl4 + 3 SnMe4 → 4 Me3SnCl
This is the Kocheshkov redistribution reaction. It is performed under an inert atmosphere, such as argon, typically with no solvent.
A second route to Me3SnCl involves treating the corresponding hydroxide or oxide with a halogenating agent such as hydrogen chloride or thionyl chloride (SOCl2):
- Me3SnOH + HCl → Me3SnCl + H2O
Uses
Trimethyltin chloride is used as a source of the trimethylstanyl group.[3] For example, it is a precursor to vinyltrimethylstannane:
- CH2=CHMgBr + Me3SnCl → Me3SnCH=CH2
Another example of a Grignard reagent reacting with Me3SnCl to form a tin-carbon bond is:
- LiCH(SiMe3)(GeMe3) + Me3SnCl → Me3SnCH(SiMe3)(GeMe3) + LiCl
Organotin compounds derived from Me3SnCl are useful in organic synthesis, especially in radical chain reactions. Me3SnCl is a precursor to compounds used in PVC stabilization. Reduction of trimethyltin chloride gives tin-tin bonds.
- Me3SnM + Me3SnCl → Sn2Me6 + MCl (M = metal)
References
- ^ Lide, David R & Milne, G.W.A. Handbook of Data on Organic Compounds. 3rd Edition. Volume IV. CRC Press: 1994. pg 4973.
- ^ Scott, William J. Crisp, G. T., Stille, J. K. (1993), "Palladium-catalyzed Coupling of Vinyl Triflates with Organostannanes: 4-tert-Butylcyclohexen-1-yl)-2-propen-1-one", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv8p0097; Coll. Vol. 8: 97
- ^ Davies, A.G. "Tin Organometallics". Comprehensive Organometallic Chemistry III. Elsevier B.V.: 2008. pg 809-883 doi:10.1016/B0-08-045047-4/00054-6
Categories:- Chlorides
- Metal halides
- Organotin compounds
Wikimedia Foundation. 2010.
