- Stannin
Pfam_box
Symbol = SNN_transmemb
Name =
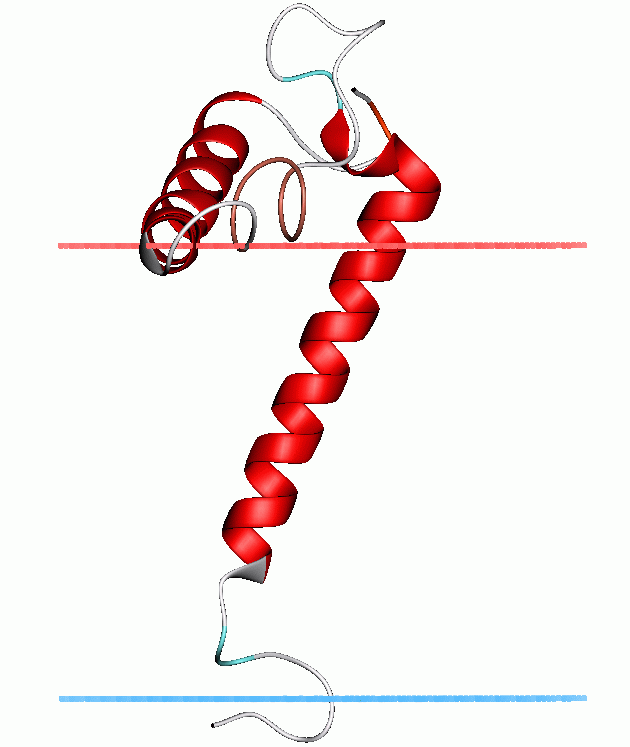
width =200
caption = Stannin
Pfam= PF09049
InterPro= IPR015135
SMART=
Prosite =
SCOP =
TCDB =
OPM family= 180
OPM protein= 1zza
PDB=PDB3|1zzaA:1-33Stannins are small proteins that consist of a single transmembrane helix, an unstructured linker domain, and a cytoplasmic domain. The transmembrane region contains a conserved cysteine residue (Cys32) that, together with Cys34found in the stannin unstructured linker domain, constitutes theputative
trimethyltin -binding site, close to the lipid/solvent interface cite journal |author=Mascioni A, Veglia G, Buck-Koehntop BA, Buffy JJ |title=Structure, dynamics, and membrane topology of stannin: a mediator of neuronal cell apoptosis induced by trimethyltin chloride |journal=J. Mol. Biol. |volume=354 |issue=3 |pages=- |year=2005 |pmid=16246365] .The unstructured protein region connects two adjacent helical domains. It contains a conserved CXC metal-binding motif and a putative 14-3-3-zeta binding domain. Upon coordinating dimethytin, considerable structural or dynamic changes in the flexible loop region of SNN may take place, recruiting other binding partners such as 14-3-3-zeta, and thereby initiating the apoptotic cascadecite journal |author=Mascioni A, Veglia G, Buck-Koehntop BA, Buffy JJ |title=Structure, dynamics, and membrane topology of stannin: a mediator of neuronal cell apoptosis induced by trimethyltin chloride |journal=J. Mol. Biol. |volume=354 |issue=3 |pages=- |year=2005 |pmid=16246365] .
The cytoplasmic domain forms a distorted helix that is partially absorbed into the plane of the lipid bilayer. It interacts with the surface of the lipid bilayer, and contributes to the initiation of the
apoptotic cascade on binding of the unstructured linker domain to dimethyltincite journal |author=Mascioni A, Veglia G, Buck-Koehntop BA, Buffy JJ |title=Structure, dynamics, and membrane topology of stannin: a mediator of neuronal cell apoptosis induced by trimethyltin chloride |journal=J. Mol. Biol. |volume=354 |issue=3 |pages=- |year=2005 |pmid=16246365] .Human proteins containing this domain
SNN ;References
Wikimedia Foundation. 2010.
