- Non-innocent ligand
-
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is unclear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.
Contents
Redox Reactions of Complexes Containing Innocent vs. Non-Innocent Ligands
Conventionally, redox reactions of coordination complexes are assumed to be metal-centered. The reduction of MnO4- to MnO42- is described by the change in oxidation state of manganese from 7+ to 6+. The oxide ligands do not change in oxidation state, remaining 2- (a more careful examination of the electronic structure of the redox partners reveals however that the oxide ligands are affected by the redox change). Oxide is an innocent ligand. Another example of conventional metal-centered redox couple is [Co(NH3)6]3+/[Co(NH3)6]2+. Ammonia is innocent in this transformation.
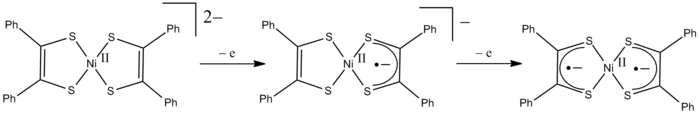
A clear example of redox non-innocent behavior of ligands is observed for [Ni(S2C2Ph2)2]z, which exists in three oxidation states: z = 2-, 1-, and 0. If the ligands are always considered to be dianionic (as is done in formal oxidation state counting), then z = 0 requires that that nickel has a formal oxidation state of +IV. The formal oxidation state of the central nickel atom therefore ranges from +II to +IV in the above transformations (see Figure). However, the formal oxidation state is different from the real (spectroscopic) oxidation state based on the (spectroscopic) metal d-electron configuration. The stilbene-1,2-dithiolate behaves as a redox non-innocent ligand, and the oxidation processes actually take place at the ligands rather than the metal. This leads to the formation of ligand radical complexes. The charge-neutral complex (z =0) is therefore best described as a Ni2+ derivative of S2C2Ph2-. The diamagnetism of this complex arises from anti-ferromagnetic coupling between the unpaired electrons of the two ligand radicals.
The complex Cr(2,2'-bipyridine)3 is a derivative of Cr(III) bound to three radical anions of 2,2'bipyridine, which is in this case also behaving as a redox non-innocent ligand. On the other hand, one-electron oxidation of [Ru(2,2'-bipyridine)3]2+ is localized on Ru and the bipyridine is behaving as a normal, innocent ligand in this case.
History
C.K. Jørgenson (Cologny-Geneva) described ligands as "innocent" and "suspect": "Ligands are innocent when they allow oxidation states of the central atoms to be defined. The simplest case of a suspect ligand is NO..."[1]
Redox non-innocent ligands have been intensively investigated spectroscopically by the groups of K. Wieghardt (MPI Mülheim a/d Ruhr) and W. Kaim (Stuttgart) over the past years. Quite recently it became obvious that redox non-innocent ligands are not just a spectroscopic curiosity, as the radical reactivity of redox non-innocent ligands was demonstrated to play a crucial role in the mechanism of bio-catalytic processes mediated by several metallo-enzymes (e.g. Gallactose Oxidase, Cytochrome P450, methane mono-oxygenase). More recently, some synthetic research groups have started to systematically investigate the (catalytic) reactivity of transition metal complexes with redox non-innocent ligands in organometallic chemistry.
Typical Ligands that often behave as Redox Non-Innocent Ligands
- O2 and NO.[2]
Ligands with extended pi-delocalization such as porphyrins and phthalocyanines, ligands with the generalised formulas [D-CR=CR-D]2- or D=CR-CR=D (D = O, S, NR’ and R, R' = alkyl or aryl), and similar related systems are often non-innocent. For example:
- dioxalenes, such as catecholates.[3]
- dithiolenes, such as 1,2-maleonitriledithiolate
- diimines such as derivatives of 1,2-diaminobenzene, α-diimines, and dimethylglyoxime.
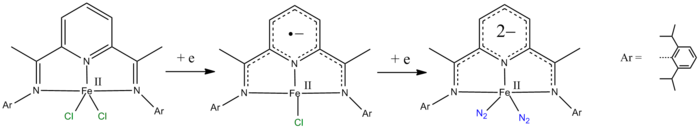
- pyridine-2,6-diimine ligands (relevant in polymerisation and hydrogenation catalysis).
The pyridine-2,6-diimine ligand can be easily reduced by one or two electrons.[4][5][6]
Redox Non-Innocent Ligands in Organometallic Chemistry and Catalysis
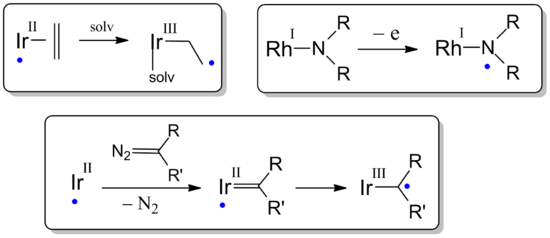
In paramagnetic organometallic complexes of Rh and Ir (metallo-radicals),[7] ethene ligands, amido ligands, and (reactive) carbene ligands are sometimes also behaving as 'redox non-innocent' ligands:
- Solvent coordination to some metallo-radical IrII(ethene) species transfers the spin-density from the metal to the redox non-innocent ethene ligand, after which direct radical coupling reactions with the olefinic ligand radical become possible.[8][9][10]
- Oxidation of certain RhI-amido and IrI-amido complexes does not lead to the expected MII-amido species. Instead the unexpected MI-aminyl radical complexes are formed.[11]
- Carbene formation from diazo compounds at metallo-radical IrII species unexpectedly leads to formation of 'carbene radicals'. This is a result of the redox non-innocent character of Fischer-type carbenes, where one-electron reduction of the carbene ligand by IrII leads to formation of carbon centered 'carbene radicals' coordinated to IrIII. These 'carbene radicals' reveal interesting radical-type reactivities.[12]
Redox Non-Innocent Ligands in Biology
Metalloenzymes often feature non-innocent ligands. A common non-innocent ligand is found in metalloporphyrins. In the enzyme cytochrome P450, the porphyrin ligand sustains oxidation during the catalytic cycle. In other heme proteins, such as myoglobin, ligand-centered redox does not occur and the porphyrin is innocent.
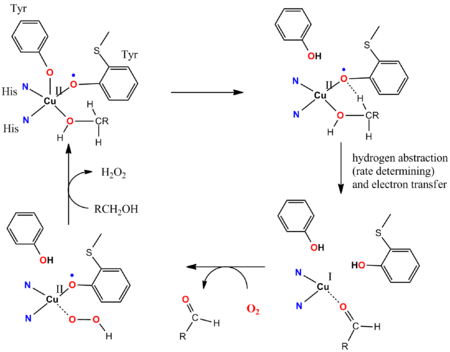
Galactose Oxidase (GOase) provides a seminal example for the involvement of reactive non-innocent ligands in bio-catalytic turnover.[13][14] GOase converts chemo-selectively primary alcohols with O2 into aldehydes and H2O2, with impressive turnover frequencies. The active site of the enzyme GOase contains a tyrosinyl radical which is coordinated to a CuII ion. In the key steps of the catalytic cycle, a cooperative Brønsted-basic ligand-site deprotonates the alcohol, and subsequently the oxygen atom of the tyrosinyl radical abstracts a hydrogen atom from the alpha-CH functionality of the coordinated alcoholate substrate. Thus, the tyrosinyl radical is a reactive fragment in the catalytic cycle which cooperates with the Cu site. This is essential for the function of the enzyme, because the Cu-ion is only capable of one-electron transformations. It is the interplay of the 1e reactivity of the ligand radical and the 1e reactivity of the metal which makes the overall process possible. The radical abstraction nature of the process makes the process extremely fast. Anti-ferromagnetic coupling between the unpaired spins of the tyrosine radical ligand and the d9 CuII ion (open-shell singlet ground state) explains the observed diamagnetic nature of the resting state of the enzyme, as was confirmed by synthetic model studies.[15]
See also
- Electromerism
- Isomerism
- Redox
References
- ^ Jørgensen, Chr. K. (1966). "Differences between the four halide ligands, and discussion remarks on trigonal-bipyramidal complexes, on oxidation states, and on diagonal elements of one-electron energy". Coordination Chemistry Reviews 1 (1-2): 164–178. doi:10.1016/S0010-8545(00)80170-8.
- ^ Kaim, W.; Schwederski, B. (2010). "Non-innocent ligands in bioinorganic chemistry—An overview". Coordination Chemistry Reviews. 254 (13-14) (13-14): 1580–1588. doi:10.1016/j.ccr.2010.01.009.
- ^ Piero Zanello, P.; Corsini, M. (2006). "Homoleptic, mononuclear transition metal complexes of 1,2-dioxolenes: Updating their electrochemical-to-structural (X-ray) properties". Coordination Chemistry Reviews 250 (15-16): 2000–2022. doi:10.1016/j.ccr.2005.12.017.
- ^ de Bruin, B.; Bill, E.; Bothe, E.; Weyhermüller, T.; Wieghardt, K. (2000). "Molecular and Electronic Structures of Bis(pyridine-2,6-diimine)metal Complexes [ML2](PF6)n(n = 0, 1, 2, 3; M = Mn, Fe, Co, Ni, Cu, Zn)". Inorganic Chemistry 39 (13): 2936–2947. doi:10.1021/ic000113j.
- ^ Budzelaar, P.H.M.; de Bruin, B.; Gal, A.W.; Wieghardt, K.; van Lenthe, J.H. (2001). "Metal-to-Ligand Electron Transfer in Diiminopyridine Complexes of Mn−Zn. A Theoretical Study". Inorganic Chemistry 40 (18): 4649–4655. doi:10.1021/ic001457c.
- ^ Chirik, P.J.; Wieghardt, K. (2010). "Radical Ligands Confer Nobility on Base-Metal Catalysts". Science. 327 (5967) (5967): 794–795. doi:10.1126/science.1183281. PMID 20150476.
- ^ de Bruin, B.; Hetterscheid, D.G.H.; Koekkoek, A.J.J.; Grützmacher, H. (2007). 5. In Karlin, Kenneth D.. "The Organometallic Chemistry of Rh-, Ir-, Pd-, and Pt-Based Radicals: Higher Valent Species". Progress in Inorganic Chemistry 55: 247–354. doi:10.1002/9780470144428.
- ^ Hetterscheid, D.G.H.; Kaiser, J.; Reijerse, E.; Peters, T.P.J.; Thewissen, S.; Blok, A.N.J.; Smits, J.M.M.; de Gelder, R.; de Bruin, B. (2005). "IrII(ethene): Metal or Carbon Radical?". Journal of the American Chemical Society 127 (6): 1895–1905. doi:10.1021/ja0439470. PMID 15701024.
- ^ Hetterscheid, D.G.H.; Bens, M.; de Bruin, B. (2005). "IrII(ethene): Metal or Carbon Radical? Part II: Oxygenation via iridium or direct oxygenation at ethene?". Dalton Transactions (5): 979–984. doi:10.1039/b417766e. PMID 15726153.
- ^ de Bruin, B.; Hetterscheid, D.G.H. (2007). "Paramagnetic (Alkene)Rh and (Alkene)Ir Complexes: Metal or Ligand Radicals?". European Journal of Inorganic Chemistry 2 (2): 211–230. doi:10.1002/ejic.200600923.
- ^ Büttner, T.; Geier, J.; Frison, G.; Harmer, J.; Calle, C.; Schweiger, A.; Schönberg, H.; Grützmacher, H. (2005). "A Stable Aminyl Radical Metal Complex". Science. 307 (5707) (5707): 235–238. doi:10.1126/science.1106070. PMID 15653498.
- ^ Dzik, W.I.; Reek, J.N.H.; de Bruin, B. (2008). "Selective C-C Coupling of Ir-Ethene and Ir-Carbenoid Radicals". Chemistry: A European Journal 14 (25): 7594–7599. doi:10.1002/chem.200800262. PMID 18523935.
- ^ Whittaker, M.M.; Whittaker, J.W. (1993). "Ligand interactions with galactose oxidase: mechanistic insights". Biophysical Journal. 64 (3): 762–772.
- ^ Wang, Y.; DuBois, J. L.; Hedman, B.; Hodgson, K. O.; Stack, T. D. P. (1998). "Catalytic Galactose Oxidase Models: Biomimetic Cu(II)-Phenoxyl-Radical Reactivity". Science. 279 (5350) (5350): 537–540. doi:10.1126/science.279.5350.537.
- ^ Müller, J.; Weyhermüller, T. Bill, E.; Hildebrandt, P.; Ould-Moussa, L.; Glaser, T.; Wieghardt, K. (1998). "Why Does the Active Form of Galactose Oxidase Possess a Diamagnetic Ground State?". Angewandte Chemie International Edition. 37 (5) (5): 616–619. doi:10.1002/(SICI)1521-3773(19980316)37:5<616::AID-ANIE616>3.0.CO;2-4.
Categories:- Coordination compounds
- Organometallic chemistry
- Chemical bonding
Wikimedia Foundation. 2010.