- Diketimine
-
This article is about the class of compounds having two imine groups. For compound having the molecular formula N2H2 and often called "diimine", see diazene.
Diketimines or diimines are a family of ligands and ligand precursors derived from 1,2- and 1,3-diketones by replacement of the carbonyl oxygen atoms with NR groups, where R = aryl, alkyl. Two families of diketimines are important in coordination chemistry and catalysis: 1,2-diketimines and 1,3-diketimines.
Contents
Preparation
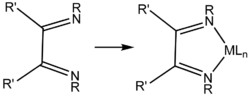
Main article: ImineDiketimines are prepared by conventional condensation reactions that are used to convert aldehydes and ketones to imines, Schiff bases, and oximes. For example, acetylacetone (2,4-pentanedione) and a primary alkyl- or arylamine will react, typically in acidified ethanol, to form a diketimine. 1,3-Diketimines derived from bulky amines, e.g. 2,6-disubstituted anilines, require prolonged reaction times.[1] 1,3-Diketimines are often referred to as "HNacNac," a modification of the abbreviation Hacac used for α,β-diketones. These species can exist as a mixture of two tautomers.
 Tautomers of a substituted HNacNac ligand precursor and an idealized complex (right) of the conjugate base (M = metal, L = other ligand).
Tautomers of a substituted HNacNac ligand precursor and an idealized complex (right) of the conjugate base (M = metal, L = other ligand).
Coordination complexes
The 1,2-diketimine ligands, also called α-diimines, include dimethylglyoxime as well as oxidized derivatives of o-phenylenediamine. The steric properties of the substituents on nitrogen provide a means to control the axial coordination sites on a square planar complex. Large planar substituents such as mesityl tend to be orthogonal to the MN2 plane. In this way, the axial coordination sites on a square planar complex are shielded. Such steric control is not possible for complexes of the related to 2,2'-bipyridine, glyoximate, and 9,10-phenanthroline ligands.
Deprotonation of HNacNac compounds affords anionic bidentate ligands that form a variety of coordination complexes.[2] Some derivatives with large R groups can been used to stabilize low valent main group and transition metal complexes.[3] Unlike the situation for the acetylacetonates, the steric properties of the coordinating atoms in NacNac- ligands is adjustable by changes in the R substituent. Attachment to a metal center is usually carried out by initial deprotonation of HNacNac with butyllithium; the lithium derivative is then treated with a metal chloride to eliminate LiCl. In some cases, HNacNacs also serve as charge-neutral 1,3-diimine ligands.
1,2-Diketimines, but not the 1,3-diketimines, are “non-innocent ligands”, akin to the dithiolenes.
Uses
Substituted α-diimine and NacNac ligands are useful in the preparation of so-called post-metallocene catalysts for the polymerization and copolymerization of ethylene and alkenes.[1][4]
References
- ^ a b Feldman, J.; McLain, S. J.; Parthasarathy, A.; Marshall, W. J.; Calabrese, J. C.; Arthur, S. D. “Electrophilic Metal Precursors and α-Diimine Ligand for Nickel(II)- and Palladium(II)-Catalyzed Ethylene Polymerization” Organometallics 1997 volume 16, pages 1514-1516. doi:10.1021/om960968x
- ^ Bourget-Merle, L.; Lappert, M. F.; Severn, J. R. “The Chemistry of -Diketiminatometal Complexes” Chemical Reviews 2002, volume 102, pages 3031-3066. doi:10.1021/cr010424r
- ^ Qian, B.; Ward, D. L.; Smith, M.R. Synthesis, Structure, and Reactivity of β-Diketiminato Aluminum Complexes Organometallics; 1998;17, pp 3070 - 3076.
- ^ Ittel, S. D.; Johnson, L. K.; Brookhart, M. “Late-Metal Catalysts for Ethylene Homo- and Copolymerization” Chemical Reviews 2000, volume 100, pages 1169-1203. doi:10.1021/cr9804644
Categories:- Coordination compounds
Wikimedia Foundation. 2010.