- Sodium maleonitriledithiolate
-
Sodium maleonitriledithiolate 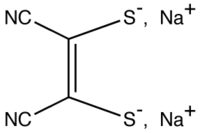 sodium cis-1,2-dicyano-1,2-ethylenedithiolateOther namessodium mnt sodium maleonitriledithiolate
sodium cis-1,2-dicyano-1,2-ethylenedithiolateOther namessodium mnt sodium maleonitriledithiolateIdentifiers PubChem 6523934 ChemSpider 5013443 
Jmol-3D images Image 1 - [Na+].[Na+].[S-]/C(C#N)=C(/[S-])C#N
Properties Molecular formula C4N2Na2S2 Molar mass 186.17 g/mol Appearance yellow solid Solubility in ethanol, DMF Soluble  maleonitriledithiolate (verify) (what is:
maleonitriledithiolate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium maleonitriledithiolate is the chemical compound described by the formula Na2S2C2(CN)2. The name refers to the cis compound, formally derived from maleonitrile. The dianion is a "dithiolene", i.e. a chelating alkene-1,2-dithiolate that serves as a non-innocent ligand for transition metals. Several complexes are known, such as [Ni(mnt)2]2-.
The salt is synthesized by treating carbon disulfide with sodium cyanide to give the cyanodithioformate salt, which eliminates elemental sulfur in aqueous solution:[1]
- 2 NaCN + 2 CS2 → Na2S2C2(CN)2 + 1/4 S8
The compound was first described by Bähr and Schleitzer 1958.[2]
References
- ^ R. H. Holm, A. Davison "Metal Complexes Derived from cis-1,2-Dicyano-1,2-Ethylenedithiolate and Bis(trifluoromethyl)-1,2-Dithiete" Inorganic Syntheses 1967, volume X, pp.8-26.
- ^ G. Bähr and G. Schleitzer (1957). "Beiträge zur Chemie des Schwefelkohlenstoffs und Selenkohlenstoffs, II. Die Kondensierende Spontan-Entschwefelung von Salzen und Estern der Cyan-Dithioameisensäure. Freie Cyan-Dithioameisensäure". Chemische Berichte 90 (3): 438–443. doi:10.1002/cber.19570900322.
Categories:- Thiols
- Alkenes
Wikimedia Foundation. 2010.
