- Conjugated linoleic acid
-
c9, t11 conjugated linoleic acid 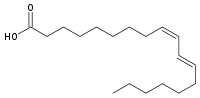 (9Z,11E)-octadeca-9,11-dienoic acidOther names
(9Z,11E)-octadeca-9,11-dienoic acidOther namesIdentifiers CAS number 2540-56-9 PubChem 5280644 ChemSpider 4444245 Jmol-3D images Image 1 - O=C(O)CCCCCCC/C=C\C=C\CCCCCC
- InChI=1/C18H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h7-10H,2-6,11-17H2,1H3,(H,19,20)/b8-7+,10-9-
Key: JBYXPOFIGCOSSB-GOJKSUSPBK
Properties Molecular formula C18H32O2 Molar mass 280.44548 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Conjugated linoleic acids (CLA) are a family of at least 28[1] isomers of linoleic acid found mainly in the meat and dairy products derived from ruminants. As the name implies, the double bonds of CLAs are conjugated, with only one single bond between them.
Contents
History
In 1979, researchers from the University of Wisconsin applied a beef extract to mice skin. The mice were then exposed to a strong carcinogen. When the researchers counted the number of tumors developed by the mice 16 weeks later, they found, to their surprise, that the mice exposed to the beef extract had 20% fewer tumors. The identity of this anticarcinogen was not discovered until almost a decade later, in 1987. Michael Pariza, the scientist who discovered CLA, later remarked that "few anticarcinogens, and certainly no other known fatty acids, are as effective as CLA in inhibiting carcinogenesis in these models." [2][3] Although CLA is best known for its anticancer properties, researchers have also found that the cis-9, trans-11 form of CLA can reduce the risk for cardiovascular disease and help fight inflammation. [4][5]
The Nutritional Immunology and Molecular Medicine Laboratory (NIMML) made a seminal discovery demonstrating that oral CLA treatment prevents or ameliorates inflammatory bowel disease by activating the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR gamma).
CLA is also known for its body weight management properties, which include reducing body fat and increasing lean muscle mass. Over 30 clinical studies have been published investigating the effect of CLA on weight management. The trials have quite variable designs, which leads to inconsistency. However a meta-analysis conducted in 2007 concluded CLA has a small impact on fat mass. [6]
In July 2008, CLA received a no objection letter from the FDA on its Generally Recognized as Safe (GRAS) status for certain food categories, including fluid milk, yogurt, meal replacement shakes, nutritional bars, fruit juices and soy milk. With GRAS status, food companies are now able to add CLA to products in these food categories.
Biochemistry
Most studies of CLAs have used a mixture of isomers wherein the isomers c9,t11-CLA and t10,c12-CLA were the most abundant.[7] More recent studies using individual isomers indicate that the two isomers have very different health effects. [4] [8]
Conjugated linoleic acid is both a trans fatty acid and a cis fatty acid. The cis bond causes a lower melting point and ostensibly also the observed beneficial health effects. Unlike other trans fatty acids, it may have beneficial effects on human health.[9] CLA is conjugated, and in the United States, trans linkages in a conjugated system are not counted as trans fats for the purposes of nutritional regulations and labeling. CLA and some trans isomers of oleic acid are produced by microorganisms in the rumens of ruminants. Non-ruminants, including humans, produce certain isomers of CLA from trans isomers of oleic acid, such as vaccenic acid, which is converted to CLA by delta-9-desaturase.[10][11]
Diet and health
Anticancer properties have been attributed to CLA, and studies on mice and rats show encouraging results in hindering the growth of tumors in mammary, skin, and colon tissues.[12][13][14][15][16][17][18][19][20][21][22][23][24][25][26] It has been reported that CLA can up-regulate the tumor suppressor gene PTPRG, and may have anti-cancer properties.[13][27]
A European team led by the Swiss scientist Lukas Rist has found that mothers consuming mostly organic milk and meat products have about 50 percent higher levels of rumenic acid in their breast milk.[28]
According to studies that targeted the effects of conjugated linoleic acid on the belly firmness and fatty acid composition of genetically lean pigs, the supplemental CLA usage had a positive effect on the improvement of belly firmness and may provide a nutritional solution to carcass fat and belly firmness problems.[29]
The most promising science around CLA concerns its effect on weight management. Thirty-five intervention studies have been conducted using CLA in humans to investigate the effects of CLA on weight management. These studies, which vary widely in CLA dose and duration, show the most significant effect of CLA on weight management is on body composition, a reduction in total body fat and an increase in lean body mass. The effect of CLA on fat mass is modest and at the recommended dosage of 3.2g/day produces a statistically significant 90 g fat loss per week (ca. 1 lb in 5 weeks) as shown by a 2007 meta-analysis[30]. Doses higher than the recommended 3.2g do not seem to have any additional effects on body fat reduction. Another meta-analysis found that CLA supplementation produces about 1% increase in lean body mass per week. With the simultaneous decrease in fat mass coupled with increases in lean body mass, often the net change in weight is small. However, the effects of CLA on body composition is a healthy effect, since the degree of fat mass is related to all cause mortality [31] and lean body mass burns more calories than fat mass which may help to increase resting metabolic rates. CLA use itself is not an answer to the prevalence of obesity, but it can be a useful tool in addition to a healthy lifestyle and exercise program to achieve and maintain a healthy body weight.
Some studies have found no significant effects of CLA supplementation on fat mass loss [32][33][34][35][36][37]. These results are likely due to a number of reasons. The study duration may have been too short to observe significant effects. The instrumentation used may not have been sensitive enough to detect significant fat losses [34][35][36]. CLA has also been used in combination with other ingredients which may skew results [38].
Possible adverse effects of CLA supplements in humans
There are concerns that the use of CLA supplements by extremely overweight people may tend to cause or to aggravate insulin resistance, which may increase their risk of developing diabetes.[8] Commercially available supplements contain equal mixtures of two CLA isomers: the cis-9, trans-11 isomer (also known as rumenic acid, the predominant CLA isomer in milk and beef), as well as the trans-10, cis-12 (t10c12) isomer. All other isomers ratios found in the scientific literature are not commercially available. The trans-10, cis-12 isomer is linked to many adverse side effects. Research indicates that supplementation with t10c12 CLA dramatically increases rates of oxidative stress, to levels considerably higher than that observed in heavy smokers.[8] However, the evidence is controversial, and some studies using a mixture of c9t11 and t10c12 CLA showed no changes in insulin sensitivity.[39][40] A study in 2007 used the euglycemic hyperinsulinemic clamp method, which is the gold standard, to evaluate insulin resistance. The study performed on 49 obese or overweight individuals taking 3.2g CLA per day for six months found no adverse effects on blood glucose management. In addition, the long term studies of one and two years have found CLA supplementation to be safe with no outstanding adverse events [41].In one study, t10c12 CLA produced a 32% increase in biliary cholesterol concentration, which increases the chance of gallstone formation [42].
In 2006, a study by the US Department of Agriculture suggested CLA can induce essential fatty acid redistribution in mice. Changes in docosahexaenoic acid (DHA) and arachidonic acid (AA) levels were observed in some organs. For instance, the t10,c12 CLA reduced the DHA content of heart tissue by 25%, while in the spleen, DHA content rose, and AA fell. DHA is an omega-3 fatty acid important to cardiovascular health, and the dramatic reduction of DHA in heart tissue can have serious health consequences. In contrast, c9,t11 CLA did not alter DHA content in the heart, but did reduce spleen DHA slightly.[7] A study of CLA supplementation (equal amounts of c9,t11 and t10,c12) in hatchling chicks (2005) showed high mortality and low hatchability rates among CLA-supplemented groups, and also a decrease in brain DHA levels of CLA-treated chicks [1]. These studies raise the question of whether CLA may increase the risk of cardiovascular and inflammatory diseases, but it has yet to be established whether such changes occur in humans, and whether they are clinically relevant.
The general consensus is that the use of this supplement should be carefully examined if the person using the supplement is greatly overweight. [43]
Dietary sources
Kangaroo meat may have the highest concentration of CLA.[44] Food products from grass-fed ruminants (e.g. mutton and beef) are good sources of CLA, and contain much more of it than those from grain-fed animals.[45] In fact, meat and dairy products from grass-fed animals can produce 300-500% more CLA than those of cattle fed the usual diet of 50% hay and silage, and 50% grain.[46]
Eggs are also rich in CLA, and CLA in eggs has been shown to survive the temperatures encountered during frying.[47]
Some mushrooms, such as Agaricus bisporus and Agaricus subrufescens, are rare nonanimal sources of CLA.[48][49]
See also
References
- ^ Banni S (June 2002). "Conjugated linoleic acid metabolism". Curr. Opin. Lipidol. 13 (3): 261–6. doi:10.1097/00041433-200206000-00005. PMID 12045395. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0957-9672&volume=13&issue=3&spage=261.
- ^ Ha YL, Grimm NK, Pariza MW (1987). "Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid". Carcinogenesis 8 (12): 1881–7. doi:10.1093/carcin/8.12.1881. PMID 3119246.
- ^ Williams, Lane; Publishing, Woodland (1999-01). CLA: Conjugated Linoleic Acid - Google Book Search. Woodland Publishing. ISBN 9781580540087. http://books.google.com/?id=su_k_WkP0KgC&dq=Conjugated+linoleic+acid+Williams&printsec=frontcover.
- ^ a b Tricon S, Burdge GC, Kew S et al. (September 2004). "Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans". Am. J. Clin. Nutr. 80 (3): 614–20. PMID 15321800.
- ^ Zulet MA, Marti A, Parra MD, Martínez JA (September 2005). "Inflammation and conjugated linoleic acid: mechanisms of action and implications for human health". J. Physiol. Biochem. 61 (3): 483–94. doi:10.1007/BF03168454. PMID 16440602.
- ^ Whigham L et al. (January 2007). "Efficacy of conjugated linoleic acid for reducing fat mass:a meta-analysis in humans". Am. J. Clin. Nutr. 85 (5): 1203–11. PMID 17490954.
- ^ a b "Fatty Acid Profiles of Liver, Adipose Tissue, Speen, and Heart of Mice Fed Diets Containing T10, C-12-, and C9, T11-Conjugated Linoleic Adic". http://www.ars.usda.gov/research/publications/publications.htm?seq_no_115=179685.
- ^ a b c Ulf Risérus, MMed; Samar Basu, PhD; Stefan Jovinge, MD, PhD; Gunilla Nordin Fredrikson, PhD; Johan Ärnlöv, MD; Bengt Vessby, MD, PhD (September 2002). "Supplementation With Conjugated Linoleic Acid Causes Isomer-Dependent Oxidative Stress and Elevated C-Reactive Protein". American Heart Association Journals 106 (15): 1925–9. doi:10.1161/01.CIR.0000033589.15413.48. PMID 12370214. 01.CIR.0000033589.15413.48v1. http://intl-circ.ahajournals.org/cgi/content/short/106/15/1925. Retrieved 2007-02-19.
- ^ II International Congress on CLA from Experimental Models to Human Application
- ^ Kuhnt K, Kraft J, Moeckel P, Jahreis G (April 2006). "Trans-11-18 : 1 is effectively Delta9-desaturated compared with trans-12-18 : 1 in humans". Br J Nutr. 95 (4): 752–761. doi:10.1079/BJN20051680. PMID 16571155.
- ^ Banni S, Angioni E, Murru E, Carta G, Melis M, Bauman D, Dong Y, Ip C (2001). "Vaccenic acid feeding increases tissue levels of conjugated linoleic acid and suppresses development of premalignant lesions in rat mammary gland". Nutr Cancer 41 (1–2): 91–7. doi:10.1207/S15327914NC41-1&2_12 (inactive 2010-09-04). PMID 12094634.
- ^ Belury, M.A. (October 2002). "Inhibition of carcinogenesis by conjugated linoleic acid: Potential mechanisms of action". Journal of Nutrition 132 (10): 2995–2998. PMID 12368384.
- ^ a b Amarù DL, Field CJ. (2009). "Conjugated Linoleic Acid Decreases MCF-7 Human Breast Cancer Cell Growth and Insulin-Like Growth Factor-1 Receptor Levels". Lipids 26 (5): 449–58. doi:10.1007/s11745-009-3288-4. PMID 19266226.
- ^ Lee Y, Thompson JT, de Lera AR, Vanden Heuvel JP. (2008). "Isomer-specific effects of conjugated linoleic acid on gene expression in RAW 264.7". J Nutr Biochem 26 (11): 848–59, 859.e1–5. doi:10.1016/j.jnutbio.2008.07.013. PMID 18993052.
- ^ Coakley M, Banni S, Johnson MC, Mills S, Devery R, Fitzgerald G, Paul Ross R, Stanton C. (2009). "Inhibitory Effect of Conjugated alpha-Linolenic Acid from Bifidobacteria of Intestinal Origin on SW480 Cancer Cells". Lipids 44 (3): 249–56. doi:10.1007/s11745-008-3269-z. PMID 19048324.
- ^ Ip C, Scimeca JA, Thompson HJ. (1994). "Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources". Cancer 233 (3): 1050–4. doi:10.1002/1097-0142(19940801)74:3+<1050::AID-CNCR2820741512>3.0.CO;2-I. PMID 8039138.
- ^ Kritchevsky D. (May 2000). "Antimutagenic and some other effects of conjugated linoleic acid". Br J Nutr. 83 (5): 459–65. PMID 10953669.
- ^ Pariza MW, Park Y, Cook ME. (July 2001). "The biologically active isomers of conjugated linoleic acid". Prog Lipid Res. 40 (4): 283–98. doi:10.1016/S0163-7827(01)00008-X. PMID 11412893.
- ^ Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. (December 2006). "Biological effects of conjugated linoleic acids in health and disease". J Nutr Biochem. 17 (12): 789–810. doi:10.1016/j.jnutbio.2006.02.009. PMID 16650752.
- ^ Donnelly C, Olsen AM, Lewis LD, Eisenberg BL, Eastman A, Kinlaw WB. (2009). "Conjugated Linoleic Acid (CLA) inhibits expression of the Spot 14 (THRSP) and fatty acid synthase genes and impairs the growth of human breast cancer and liposarcoma cells". Nutr Cancer. 61 (1): 114–22. doi:10.1080/01635580802348666. PMC 2872989. PMID 19116881. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2872989.
- ^ Islam MA, Kim YS, Jang WJ, Lee SM, Kim HG, Kim SY, Kim JO, Ha YL. (2008). "A mixture of trans, trans conjugated linoleic acid induces apoptosis in MCF-7 human breast cancer cells with reciprocal expression of Bax and Bcl-2". J Agric Food Chem (Korea) 56 (14): 5970–6. doi:10.1021/jf8004977. PMID 18570428.
- ^ Meng X, Shoemaker SF, McGee SO, Ip MM. (2008). "t10,c12-Conjugated linoleic acid stimulates mammary tumor progression in Her2/ErbB2 mice through activation of both proliferative and survival pathways". Carcinogenesis (NY, USA) 29 (5): 1013–21. doi:10.1093/carcin/bgn035. PMC 2777529. PMID 18339686. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2777529.
- ^ Kim YS, Cerbo RM, Hah CK, Bahn KN, Kim JO, Ha YL. (2008). "Growth inhibition of osteosarcoma cell MG-63 by a mixture of trans,trans conjugated linoleic acid isomers: possible mechanistic actions". J Food Sci. (Korea) 73 (1): T7–15. doi:10.1111/j.1750-3841.2007.00584.x. PMID 18211379.
- ^ Kelley NS, Hubbard NE, Erickson KL. (2007). "Conjugated linoleic acid isomers and cancer". J Nutr (UC Davis, Ca, USA) 137 (12): 2599–607. PMID 18029471.
- ^ Fite A, Goua M, Wahle KW, Schofield AC, Hutcheon AW, Heys SD. (2007). "Potentiation of the anti-tumour effect of docetaxel by conjugated linoleic acids (CLAs) in breast cancer cells in vitro". Prostaglandins Leukot Essent Fatty Acids. (Scotland, UK) 77 (2): 87–96. doi:10.1016/j.plefa.2007.08.004. PMID 17900885.
- ^ Ou L, Ip C, Lisafeld B, Ip MM. (2007). "Conjugated linoleic acid induces apoptosis of murine mammary tumor cells via Bcl-2 loss". Biochem Biophys Res Commun. (NY, USA) 356 (4): 1044–9. doi:10.1016/j.bbrc.2007.03.096. PMC 1992442. PMID 17400188. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1992442.
- ^ Wang LS, Huang YW, Sugimoto Y (2006), "Conjugated linoleic acid (CLA) up-regulates the estrogen-regulated cancer suppressor gene, protein tyrosine phosphatase {gamma} (PTP{gamma}), in human breast cells.", Anticancer Res 26: 27–34, PMID 16475675
- ^ Lukas Rist, Andre Mueller, Christiane Barthel, Bianca Snijders, Margje Jansen, A. Paula Simoes-Wust, Machteld Huber, Ischa Kummeling, Ursula von Mandach, Hans Steinhart, and Carel Thijs. (June 2007). "Influence of organic diet on the amount of conjugated linoleic acids in breast milk". British Journal of Nutrition.
- ^ S. T. Larsen, B. R. Wiegand,2, F. C. Parrish, Jr., J. E. Swan and J. C. Sparks (2009). "Dietary conjugated linoleic acid changes belly and bacon quality from pigs fed varied lipid sources". Journal of Animal Science (American Society of Animal Science) 87 (1): 285–295. doi:10.2527/jas.2008-1213. PMID 18820159. http://jas.fass.org/cgi/content/full/87/1/285
- ^ * Whingham LD, Watras CA, Scholler DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr 2007;85(5):1203-1200.
- ^ * Heitmann BL, Erikson H, Ellsinger BM, Mikkelsen KL, Larrson B. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old swedish men - a 22-year follow-up. The study of men born in 1913. Int J Obes Relat Metab Disord 2000;24(1):33-37.
- ^ * Lambert EV, Goedecke JH, Bluett K, Heggie K, Claassen A, Rae DE, West S, Dugas J, Dugas L, Meltzer S, Charlton K, Mohede I. Conjugated linoleic acid versus high-oleic acid sunflower oil: effects on energy metabolim, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br J Nutr 2007;97(5):1001-1011.
- ^ * Nazare JA, de la Perriere AB, Bonnet F, Desage M , Peyrat J, Maitrepierre C, Louche-Pelissier C, Bruzeau J, Goudable J, Lassel T, Vidal H, Laville M. Daily intake of conjugated linoleic acid-enriched yoghurts:effects on energy metabolism and adipose tissue gene expression in healthy subjects. Br J Nutr 2007;97(2):273-280.
- ^ a b * Petridou A, Mougios V, Sagredos A. Supplementation with CLA: isomer incorporation into serum lipids and effect on body fat of women. Lipids 2003;38(8):805-11.
- ^ a b * Berven G, Amund B, Ottar H, Blankson H, Fagertun H, Thom E, Wadstein J, Gudmundson O. Safety of conjugated linoleic acid (CLA) in overweight or obese human volunteers. Eur J Lipid Sci Technol 2000;102:455-462.
- ^ a b * Whigham LD, O'Shea M, Mohede IC, Walaski HP, Atkinson RL. Safety profile of conjugated linoleic acids in a 12-month trial in obese humans, Food Chem Toxicol 2004; 42(10):1701-9.
- ^ * Atkinson RL et al. Conjugated linoleic acid for altering body composition and treating obesity. In: Advances in conjugated linoleic acid research, Vol. 1. Eds M.P. Yurawecz, M.M Mossoba, J.K.G. Kramer, M.W. Pariza, G.J. Nelson, AOCS Press, Champaign Illinois (USA) 1999, pp. 384-35.
- ^ * Diaz ML, Watkins BA, Li Y, Anderson RA, Campbell WW. Chromium pocolinate and conjugated linoleic acid do not synergistically influence diet- and exercise-induced changes in body composition and health indexes in overweight women. J Nutr Biochem 2008;19(1):61-68.
- ^ Watras, AC; Buchholz, AC; Close, RN; Zhang, Z; Schoeller, DA (2006-08-22). "The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain". International Journal of Obesity 31 (3): 481–7. doi:10.1038/sj.ijo.0803437. PMID 16924272. http://www.nature.com/ijo/journal/v31/n3/abs/0803437a.html. Retrieved 2008-09-30.
- ^ Syvertsen, C; Halse, J; Høivik, HO; Gaullier, JM; Nurminiemi, M; Kristiansen, K; Einerhand, A; O'Shea, M et al. (2006-10-10). "The effect of 6 months supplementation with conjugated linoleic acid on insulin resistance in overweight and obese". International Journal of Obesity 31 (7): 1148–54. doi:10.1038/sj.ijo.0803482. PMID 17031391. http://www.nature.com/ijo/journal/v31/n7/abs/0803482a.html. Retrieved 2008-09-30.
- ^ * Gaullier JM, Hals J, Hoye K, Kristiansen K, Fagertun H, Vik H, Gudmundsen O. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr 2005;135(4):778-84.
- ^ 33.^ * Nazare JA, de la Perriere AB, Bonnet F, Desage M , Peyrat J, Maitrepierre C, Louche-Pelissier C, Bruzeau J, Goudable J, Lassel T, Vidal H, Laville M. Daily intake of conjugated linoleic acid-enriched yoghurts:effects on energy metabolism and adipose tissue gene expression in healthy subjects. Br J Nutr 2007;97(2):273-280.
- ^ http://www.webmd.com/diet/news/20040520/cla-weight-loss
- ^ "Kangaroo meat - health secret revealed" (Press release). Commonwealth Scientific and Industrial Research Organisation (CSIRO). 2004-04-23. http://www.csiro.au/files/mediarelease/mr2004/kangaroofat.htm. Retrieved 2007-01-23.
- ^ T. R. Dhiman, L. D. Satter, M. W. Pariza, M. P. Galli, K. Albright, and M. X. Tolosa (1 May 2000). "Conjugated Linoleic Acid (CLA) Content of Milk from Cows Offered Diets Rich in Linoleic and Linolenic Acid". Journal of Dairy Science 83 (5): 1016–1027. doi:10.3168/jds.S0022-0302(00)74966-6. PMID 10821577. http://jds.fass.org/cgi/content/abstract/83/5/1016. Retrieved 2006-05-27.
- ^ T. R. Dhiman (2001). "Role of diet on conjugated linoleic acid content of milk and meat" (PDF). Journal of Animal Science 79. http://www.adsa.org/jointabs/iaafs108.pdf. Retrieved 2007-03-09.
- ^ Lin Yang, Ying Cao, Zhen-Yu Chen (2004). "Stability of conjugated linoleic acid isomers in egg yolk lipids during frying". Food Chemistry (Elsevier) 86 (4): 531–535. doi:10.1016/j.foodchem.2003.09.006.
- ^ Chen, S.; Oh, SR; Phung, S; Hur, G; Ye, JJ; Kwok, SL; Shrode, GE; Belury, M et al. (2006). "Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus)". Cancer Res. 66 (24): 12026–12034. doi:10.1158/0008-5472.CAN-06-2206. PMID 17178902.
- ^ W. J. Jang S. W. Hyung. Production of natural c9,t11 conjugated linoleic acid (c9,t11 CLA) by submerged liquid culture of mushrooms. Division of Applied Life Science (BK21), Graduate School, Gyeongsang National University, Jinju, 660-701, South Korea.. http://ift.confex.com/ift/2004/techprogram/paper_24802.htm.
General references
- Tokuşoğlu Ö. (2008). Conjugated linoleic acid (CLA) cis 9, trans 11 and trans 10, cis 12 isomer detection in crude and refined corn oils by capillary GC. Grasas y Aceites (Spain). Vol.59(2) Abril-Junio 2008, 146-151.
- Tokuşoğlu Ö., Durucasu İ., Akalın A.S., Serin E., Akşit S. (2007). Fatty Acid (FA) and Conjugated Linoleic Acid (CLA) Profiles of Infant Formulas Through Direct Transesterification of Acyl Lipids. Italian J Food Sci. No:4 Vol.19, 477-484.
- Akalın A.S., Tokuşoğlu Ö., Gonc S., Aycan Ş. (2007). Occurrence of conjugated linoleic acid in probiotic yoghurts supplemented with fructooligosaccharide. International Dairy Journal. Vol 17/9, 1089-1095.
- Schmid A., Collomb M., Sieber R., Bee G. Conjugated linoleic acid in meat and meat products: A review // Meat Science. – 2006. – 73. – P. 29–41.
- Jenkins T. C., McGuire M. A. Major advances in nutrition: impact on milk composition // J. Dairy Sci. – 2006. – 89 (4) – Р. 1302–1310.
- Akalın A.S., Tokuşoğlu Ö., Gönç S., Ökten S.(2005). “Detection of Biologically Active Isomers of Conjugated Linoleic Acid in "Kaymak". Grasas Aceties 56(4), 298-302.
- Al Sarakbi W, Salhab M, Mokbel K. Dairy products and breast cancer risk: a review of the literature. Int J Fertil Women's Med. 2005 Nov-Dec;50(6):244-9. Review.
- Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem. 2006 Dec;17(12):789-810. Epub 2006 May 2. Review.
- Ip MM, Masso-Welch PA, Ip C. Prevention of mammary cancer with conjugated linoleic acid: role of the stroma and the epithelium. J Mammary Gland Biol Neoplasia. 2003 Jan;8(1):103-18. Review.
- Kritchevsky D. Antimutagenic and some other effects of conjugated linoleic acid // British Journal of Nutrition. – 2000. – 83, N 5. – P. 459-465.
- Larsson S. C., Bergkvist L., Wolk A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish Mammography Cohort // American Journal of Clinical Nutrition. – 2005. – 82, N 4. – P. 894-900.
- Lee KW, Lee HJ, Cho HY, Kim YJ. Role of the conjugated linoleic acid in the prevention of cancer. Crit Rev Food Sci Nutr. 2005;45(2):135-44. Review.
- Maynard L. J., Franklin S. T. Functional foods as a value-added strategy: the commercial potential of "cancer-fighting" dairy products // Review of Agricultural Economics. – 2003. – 25, N 2. – P. 316-331.
- Miller Á., Stanton C., Murphy J., Devery R. Conjugated linoleic acid (CLA)-enriched milk fat inhibits growth and modulates CLA-responsive biomarkers in MCF-7 and SW480 human cancer cell lines // British Journal of Nutrition. – 2003. – 90, N 5. – P. 877-885.
- Pariza MW, Park Y, Cook ME. Conjugated linoleic acid and the control of cancer and obesity. Toxicol Sci. 1999 Dec;52 (2 Suppl):107-10. Review.
- Tanaka K. Occurrence of conjugated linoleic acid in ruminant products and its physiological functions // Animal Science Journal. – 2005. – 76, N 4. – P. 291-303.
- Voorrips L. E., Brants H. A. M., Kardinaal A. F. M., Hiddink G. J., Brandt P. A., van den Goldbohm R. A. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: the Netherlands cohort study on diet and cancer // American Journal of Clinical Nutrition. – 2002. – 76, N 4. – P. 873-882.
- Belury M. A. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action // Annu. Rev. Nutr. – 2002. – 22. – P. 505–531.
- Bauman D. E., Corl B. A., Baumgard L. H., Griinari J. M. Conjugated linoleic acid (CLA) and the dairy cow // In Recent Advances in Animal Nutrition / Ed. P. C. Garnsworthy, J. Wiseman; Nottingham Univ. Press. – Nottingham, UK, 2001 – P. 221–250.
- Harefoot C. G., Hazlewood G. P. Lipid metabolism in the rumen / In: Hobson P. N., Stewart C. S. (Eds.), The Rumen Microbial Ecosystem, second ed. Blackie Academic, London, 1999. – P. 382–426.
- Whingham LD, Watras CA, Scholler DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr 2007;85(5):1203-1200.
- Bassaganya-Riera, J., R. Hontecillas, D. R. Zimmerman, and, M. J. Wannemuehler. (2001) Dietary conjugated linoleic acid modulates phenotype and effector functions of porcine CD8+ lymphocytes. J. Nutr. 131: 2370-2377.
- Bassaganya-Riera, J., R. Hontecillas-Magarzo, K. Bregendahl, M. J. Wannemuehler, and D. R. Zimmerman. (2001) Effects of dietary conjugated linoleic acid in nursery pigs of dirty and clean environments on growth, empty body composition and immune competence. J. Anim. Sci. 79: 714-721.
- Bassaganya-Riera, J., R. Hontecillas, D. R. Zimmerman, and, M. J. Wannemuehler. (2001) Long-term influence of lipid nutrition on CD8+ responses to viral and bacterial antigens. Vaccine 20: 1435-1444.
- Bassaganya-Riera, J., R. Hontecillas, and, M. J. Wannemuehler. (2002) Nutritional impact of conjugated linoleic acid: a model functional food ingredient. In Vitro Cellular and Developmental Biology--Plant, 38 (3): 241-246.
- R. Hontecillas, D.L. Hutto, D. U. Ahn, J. H. Wilson, M. J. Wannemuehler,and J.Bassaganya-Riera. (2002) Nutritional regulation of bacterial-induced colitis by dietary conjugated linoleic acid. J. Nutr. 132: 2019-2027.
- Bassaganya-Riera, J., R. Hontecillas, D.C. Beitz. (2002) Colonic Anti-inflammatory Mechanisms of Conjugated Linoleic Acid. Clinical Nutrition 21 (6): 451-459.
- Bassaganya-Riera, J., Pogranichnyi, R., Jobgen, S.C., Halbur, P.G., Yoon, K-Y, O’Shea, M., Mohede, I., Hontecillas, R. (2003) CLA Ameliorates Viral Infectivity in a Pig Model of Virally Induced Immunosuppression. J. Nutr. 133: 3204-3214.
- Bassaganya-Riera, J., K. Reynolds, S. Martino-Catt, Y. Cui, L. Hennighausen, F. Gonzalez, J. Rohrer, A. Uribe Benninghoff, and R. Hontecillas (2004) Activation of peroxisome proliferator-activated receptor γ and δ by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 127: 777-791.
- O’Shea, M., J. Bassaganya-Riera, and I. Mohede. (2004) Immunomodulatory Properties of Conjugated Linoleic Acid. Am. J. Clin. Nutr. 79 (6): 1199S-1206S.
- Bassaganya-Riera, J., J. King, and R. Hontecillas. (2004) Health Benefits of CLA: Lessons from Pig Models in Biomedical Research. Eur. Journal of Lipid Science and Technology 106: 856-861.
- Bassaganya-Riera, J., and R. Hontecillas (2006) CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Clinical Nutrition. 25: 454-465.
- Evans, N.P., S. Misyak, J.L. Robertson, J. Bassaganya-Riera, R.W. Grange (2009) Immune-mediated mechanisms potentially regulate the disease time-course of duchenne muscular dystrophy and provide targets for therapeutic intervention. PM&R. 1(8): 755-768.
Categories:- Fatty acids
Wikimedia Foundation. 2010.
