- Tetralin
-
Tetralin 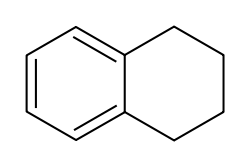 1,2,3,4-tetrahydronaphthaleneOther namesnaphthalene 1,2,3,4-tetrahydride
1,2,3,4-tetrahydronaphthaleneOther namesnaphthalene 1,2,3,4-tetrahydride
Bacticin
benzocyclohexane
THNIdentifiers CAS number 119-64-2 
PubChem 8404 ChemSpider 8097 
UNII FT6XMI58YQ 
KEGG C14114 
ChEBI CHEBI:35008 Jmol-3D images Image 1 - c1ccc2c(c1)CCCC2
Properties Molecular formula C10H12 Molar mass 132.2 g mol−1 Appearance Clear, colorless liquid Density 0.970 g/cm³ Melting point -35.8 °C, 237 K, -32 °F
Boiling point 206-208 °C, 479-481 K, 403-406 °F
Solubility in water Insoluble Hazards MSDS JT Baker MSDS Flash point 77 °C Autoignition
temperature385 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.
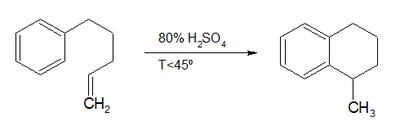
The compound can be synthesized in a Bergman cyclization. In a classic named reaction called the Darzens tetralin synthesis (Auguste George Darzens (1867–1954), 1926) derivatives can be prepared by intramolecular ring-closing reaction of an 1-aryl-4-pentene with concentrated sulfuric acid,[1] or simply through the hydrogenation of naphthalene in the presence of a platinum catalyst.
Production
Tetralin is produced by the catalytic hydrogenation of naphthalene.
A large amount of work has been devoted to the hydrogenation of naphthalene into tetralin. Mixtures containing different proportions of naphthalene, tetralin, and decalin can be produced depending on the pressure and temperature of the process. Tetralin has been obtained from pressed naphthalene isolated from coke tar by hydrogenating it over commercial catalyst WS2 + NiS + Al2O3 and CoO + MoO3 + Al2O3 under pressure of 50-300 atm. The extent to which naphthalene is hydrogenated at pressures up to 60-70 atm is determined by catalyst activity; it is maximum if CoO + MoO3 + Al2O3 or WS2 + NiS + Al2O3 catalyst are used at 300-370 °C.[2]
See also
References
- ^ Darzens Synthesis of Tetralin Derivatives
- ^ Krichko, A. A., T. A. Titova, B. S. Filippov, and N. E. Dogadkina. "Production of tetralin by the hydrogenation of naphthalene-containing fractions." Chemistry and Technology of Fuels and Oils 5 (1969): 18-22.
Categories:- Tetralins
- Alkylbenzenes
Wikimedia Foundation. 2010.