- Nitronate
-
Nitronate AzinateIdentifiers Jmol-3D images Image 1 - [O-][NH+]([O-])[O-]
- InChI=1S/HNO3/c2-1(3)4/h1H/q-2
Key: MXGJTGDTPQKTCZ-UHFFFAOYSA-N
Properties Molecular formula HNO32- Molar mass 63.0128 g mol-1 Exact mass 62.995642903 g mol-1 Structure Coordination
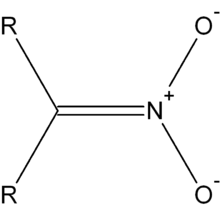
geometryTetragonal at N Molecular shape Tetrahedral at N Related compounds Other anions Nitrate Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references A nitronate (IUPAC: azinate) in organic chemistry is a functional group with the general structure R1R2C=N+(O2)2-).[1] It is the anion of a nitronic acid, a tautomeric form of a nitro compound. A nitronic acid is also called a aci form. In the Nef reaction nitronic acids are degraded to ketones. They can be alkylated on oxygen and used as a dipole in 1,3-dipolar cycloadditions.
References
- ^ F. A. Carey, R. J. Sundberg, Organische Chemie, Wiley-VCH Verlag 2004 ISBN 3-527-29217-9
Categories:- Functional groups
- Organonitrogen compounds
Wikimedia Foundation. 2010.