- Mercury(II) fulminate
-
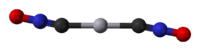
Mercury(II) fulminate 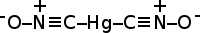

Identifiers CAS number 628-86-4 
PubChem 11022444 ChemSpider 9197626 
ChEBI CHEBI:39152 
Jmol-3D images Image 1 - [O-][N+]#C[Hg]C#[N+][O-]
Properties Molecular formula Hg(CNO)2 Molar mass 284.624 g/mol Appearance Grey Crystalline solid Density 4.43 g/cm3 Solubility in water slightly soluble Solubility soluble in ethanol, ammonia Explosive data Shock sensitivity High Friction sensitivity High Explosive velocity 4250 m/s Hazards Main hazards Highly Toxic, Shock Sensitive Explosive Autoignition
temperature180 °C  fulminate (verify) (what is:
fulminate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Mercury(II) fulminate, or Hg(CNO)2, is a primary explosive. It is highly sensitive to friction and shock. It is mainly used as a trigger for other explosives in percussion caps and blasting caps. Mercury(II) cyanate, though its formula is identical, has a different atomic arrangement; the cyanate and fulminate anions are isomers.
First used as a priming composition in small copper caps after the 1830s, mercury fulminate quickly replaced flints as a means to ignite black powder charges in muzzle loading firearms. Later, during the late 19th century and most of the 20th century, mercury fulminate or potassium chlorate became widely used in primers for self-contained rifle and pistol ammunition. Mercury fulminate has the distinct advantage over potassium chlorate of being non-corrosive, but it is known to weaken with time. Today, mercury fulminate has been replaced in primers by more efficient chemical substances. Those are non-corrosive, less toxic and more stable over time: lead azide, lead styphnate and tetrazene derivatives. In addition, none of these compounds replacing Hg(II) fulminate requires mercury for manufacture, supplies of which can be unreliable in wartime.
Preparation
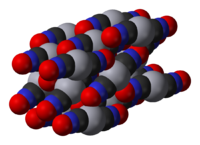
Mercury(II) fulminate is prepared by dissolving mercury in nitric acid and adding ethanol to the solution. It was first prepared by Edward Charles Howard in 1800.[1] The crystal structure of this compound was only determined in 2007.[2]
Silver fulminate can be prepared in a similar way, but this salt is even more unstable than mercury fulminate; it can even explode under water.
References
- ^ Edward Howard (1800). "On a New Fulminating Mercury". Philosophical of the Royal Society of London 90 (1): 204–238. doi:10.1098/rstl.1800.0012.
- ^ W. Beck, J. Evers, M. Göbel, G. Oehlinger and T. M. Klapötke (2007). "The Crystal and Molecular Structure of Mercury Fulminate (Knallquecksilber)". Zeitschrift für anorganische und allgemeine Chemie 633 (9): 1417–1422. doi:10.1002/zaac.200700176.
External links
Categories:- Organomercury compounds
- Fulminates
Wikimedia Foundation. 2010.
