- Equipartition theorem
[
 Thermal motion of an α-helical
Thermal motion of an α-helical peptide . The jittery motion is random and complex, and the energy of any particular atom can fluctuate wildly. Nevertheless, the equipartition theorem allows the "average" kinetic energy of each atom to be computed, as well as the "average" potential energies of many vibrational modes. The grey, red and blue spheres representatom s ofcarbon ,oxygen andnitrogen , respectively; the smaller white spheres represent atoms ofhydrogen .]In classical
statistical mechanics , the equipartition theorem is a general formula that relates thetemperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, inthermal equilibrium , energy is shared equally among its various forms; for example, the averagekinetic energy in the translational motion of a molecule should equal the average kinetic energy in itsrotational motion .The equipartition theorem makes quantitative predictions. Like the
virial theorem , it gives the "total" average kinetic and potential energies for a system at a given temperature, from which the system'sheat capacity can be computed. However, equipartition also gives the average values of "individual components" of the energy, such as the kinetic energy of a particular particle or the potential energy of a single spring. For example, it predicts that every molecule in anideal gas has an average kinetic energy of (3/2)"kBT" in thermal equilibrium, where "kB" is theBoltzmann constant and "T" is the temperature. More generally, it can be applied to any classical system inthermal equilibrium , no matter how complicated. The equipartition theorem can be used to derive the classicalideal gas law , and theDulong–Petit law for the specific heat capacities of solids. It can also be used to predict the properties ofstar s, evenwhite dwarf s andneutron star s, since it holds even when relativistic effects are considered. Although the equipartition theorem makes very accurate predictions in certain conditions, it becomes inaccurate when quantum effects are significant, namely at low enough temperatures. When the thermal energy "kBT" is smaller than the quantum energy spacing in a particular degree of freedom, the average energy and heat capacity of this degree of freedom are less than the values predicted by equipartition. Such a degree of freedom is said to be "frozen out" when the thermal energy is much smaller than this spacing. For example, thespecific heat of a solid decreases at low temperatures as various types of motion become frozen out, rather than remaining constant as predicted by equipartition. Such decreases in specific heat were the first sign to physicists of the 19th century thatclassical physics was incorrect and that new physics was needed. Along with other evidence, equipartition's failure forelectromagnetic radiation — also known as theultraviolet catastrophe — ledAlbert Einstein to suggest that light itself was quantized intophoton s, a revolutionary hypothesis that spurred the development ofquantum mechanics andquantum field theory .Basic concept and simple examples
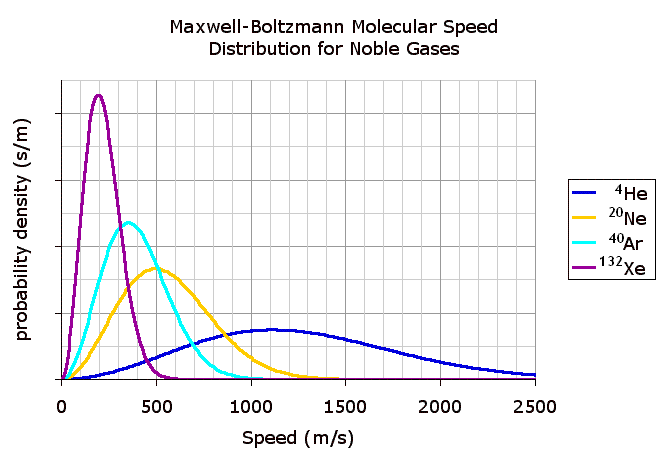
right|thumb|440px|Figure_2._Probability_density_functions_of_the_molecular_speed_for_four_noble gas es at atemperature of 298.15 K (25 °C). The four gases arehelium (4He),neon (20Ne),argon (40Ar) andxenon (132Xe); the superscripts indicate theirmass number s. These probability density functions have dimensions of probability times inverse speed; since probability is dimensionless, they can be expressed in units of seconds per meter.]The name "equipartition" means "share and share alike". The original concept of equipartition was that the total
kinetic energy of a system is shared equally among all of its independent parts, "on the average", once the system has reached thermal equilibrium. Equipartition also makes quantitative predictions for these energies. For example, it predicts that every atom of anoble gas , in thermal equilibrium at temperature "T", has an average translational kinetic energy of (3/2)"kBT", where "kB" is theBoltzmann constant . As a consequence, the heavier atoms ofxenon have a lower average speed than do the lighter atoms ofhelium at the same temperature. Figure 2 shows theMaxwell–Boltzmann distribution for the speeds of the atoms in four noble gases.In this example, the key point is that the kinetic energy is quadratic in the velocity. The equipartition theorem shows that in thermal equilibrium, any degree of freedom (such as a component of the position or velocity of a particle) which appears only quadratically in the energy has an average energy of ½"kBT" and therefore contributes ½"kB" to the system's
heat capacity . This has many applications.Translational energy and ideal gases
The (Newtonian) kinetic energy of a particle of mass "m", velocity v is given by
:
where "vx", "vy" and "vz" are the cartesian components of the velocity v. Here, "H" is short for Hamiltonian, and used henceforth as a symbol for energy because the Hamiltonian formalism plays a central role in the most general form of the equipartition theorem.
Since the kinetic energy is quadratic in the components of the velocity, by equipartition these three components each contribute ½"kBT" to the average kinetic energy in thermal equilibrium. Thus the average kinetic energy of the particle is (3/2)"kB"T, as in the example of noble gases above.
More generally, in an
ideal gas , the total energy consists purely of (translational) kinetic energy: by assumption, the particles have no internal degrees of freedom and move independently of one another. Equipartition therefore predicts that the average total energy of an ideal gas of "N" particles is (3/2) "N kBT".It follows that the
heat capacity of the gas is (3/2) "N kB" and hence, in particular, the heat capacity of a mole of a such gas particles is (3/2)"NAkB"=(3/2)"R", where "NA" isAvogadro's number and "R" is thegas constant . Since "R" ≈ 2 cal/(mol·K), equipartition predicts that themolar heat capacity of an ideal gas is roughly 3 cal/(mol·K). This prediction is confirmed by experiment.The mean kinetic energy also allows the
root mean square speed "vrms" of the gas particles to be calculated::
where "M = NAm" is the mass of a mole of gas particles. This result is useful for many applications such as
Graham's law ofeffusion , which provides a method for enrichinguranium . [ [http://www.nrc.gov/reading-rm/doc-collections/fact-sheets/enrichment.html Fact Sheet on Uranium Enrichment] U.S. Nuclear Regulatory Commission. Accessed 30th April 2007]Rotational energy and molecular tumbling in solution
A similar example is provided by a rotating molecule with
principal moments of inertia "I1", "I2" and "I3". The rotational energy of such a molecule is given by:where "ω1", "ω2", and "ω3" are the principal components of the
angular velocity . By exactly the same reasoning as in the translational case, equipartition implies that in thermal equilibrium the average rotational energy of each particle is (3/2)"kBT". Similarly, the equipartition theorem allows the average (more precisely, the root mean square) angular speed of the molecules to be calculated.The tumbling of rigid molecules — that is, the random rotations of molecules in solution — plays a key role in the relaxations observed by
nuclear magnetic resonance , particularly protein NMR andresidual dipolar coupling s. [ Bigr angle = delta_{mn} k_{B} T.
Here "δmn" is the
The general equipartition theorem holds in both the The average temperature of a star can be estimated from the equipartition theorem. [cite book | last = Chiu | first = H-Y | year = 1968 | title = Stellar Physics, volume I | publisher = Blaisdell Publishing | location = Waltham, MA | id = LCCN|67|0|17990] Since most stars are spherically symmetric, the total gravitational potential energy can be estimated by integration : where "M(r)" is the mass within a radius "r" and "ρ(r)" is the stellar density at radius "r"; "G" represents the : where "M" is the star's total mass. Hence, the average potential energy of a single particle is : where "N" is the number of particles in the star. Since most : Substitution of the mass and radius of the tar formation The same formulae may be applied to determining the conditions for : Assuming a constant density ρ for the cloud : yields a minimum mass for stellar contraction, the Jeans mass "MJ" : Substituting the values typically observed in such clouds ("T"=150 K, ρ = 2×10-16 g/cm3) gives an estimated minimum mass of 17 solar masses, which is consistent with observed star formation. This effect is also known as the Derivations Kinetic energies and the Maxwell–Boltzmann distribution The original formulation of the equipartition theorem states that, in any physical system in : for the speed of a particle of mass "m" in the system, where the speed "v" is the magnitude of the The Maxwell–Boltzmann distribution applies to any system composed of atoms, and assumes only a : as stated by the equipartition theorem.The same result can also be obtained by averaging the particle energyusing the probability of finding the particle in certain quantum energy state. Quadratic energies and the partition function More generally, the equipartition theorem states that any degree of freedom "x" which appears in the total energy "H" only as a simple quadratic term "Ax"2, where "A" is a constant, has an average energy of "½kBT" in thermal equilibrium. In this case the equipartition theorem may be derived from the partition function "Z"("β"), where "β"=1/("k""B""T") is the canonical : in the formula for "Z". The mean energy associated with this factor is given by : as stated by the equipartition theorem. General proofs General derivations of the equipartition theorem can be found in many To explain these derivations, the following notation is introduced. First, the phase space is described in terms of generalized position coordinates "q""j" together with their conjugate momenta "p""j". The quantities "q""j" completely describe the configuration of the system, while the quantities ("q""j","p""j") together completely describe its state. Secondly, the infinitesimal volume:of the phase space is introduced and used to define the volume Γ("E", Δ"E") of the portion of phase space where the energy "H" of the system lies between two limits, "E" and "E+ΔE": :In this expression, "ΔE" is assumed to be very small, "ΔE< Since "ΔE" is very small, the following integrations are equivalent : where the ellipses represent the integrand. From this, it follows that Γ is proportional to ΔE : where "ρ(E)" is the : The canonical ensemble In the : where "β = 1/kBT". : where "dΓk = dΓ/dxk", i.e., the first integration is not carried out over "xk". The first term is usually zero, either because "xk" is zero at the limits, or because the energy goes to infinity at those limits. In that case, the equipartition theorem for the canonical ensemble follows immediately : Here, the averaging symbolized by is the The microcanonical ensemble In the microcanonical ensemble, the system is isolated from the rest of the world, or at least very weakly coupled to it. Hence, its total energy is effectively constant; to be definite, we say that the total energy "H" is confined between "E" and "E+ΔE". For a given energy "E" and spread "ΔE", there is a region of : where the last equality follows because "E" is a constant that does not depend on "xn". Integrating by parts yields the relation :since the first term on the right hand side of the first line is zero (it can be rewritten as an integral of "H" - "E" on the Substitution of this result into the previous equation yields : Since the equipartition theorem follows: : Thus, we have derived the general formulation of the equipartition theorem : which was so useful in the applications described above. Limitations [ Requirement of ergodicity The law of equipartition holds only for ergodic systems in A commonly cited counter-example where energy is "not" shared among its various forms and where equipartition does "not" hold in the microcanonical ensemble is a system of coupled harmonic oscillators.cite book | last = Reichl | first = LE | year = 1998 | title = A Modern Course in Statistical Physics | edition = 2nd ed. | publisher = Wiley Interscience | id = ISBN 978-0471595205 | pages = 326–333] If the system is isolated from the rest of the world, the energy in each Failure due to quantum effects The law of equipartition breaks down when the thermal energy "kBT" is significantly smaller than the spacing between energy levels. Equipartition no longer holds because it is a poor approximation to assume that the energy levels form a smooth To illustrate the breakdown of equipartition, consider the average energy in a single (quantum) harmonic oscillator, which was discussed above for the classical case. Its quantum energy levels are given by "En = nhν", where "h" is : where "β" = 1/"k""B""T" and the denominator "Z" is the : Its average energy is given by : Substituting the formula for "Z" gives the final result : At high temperatures, when the thermal energy "kBT" is much greater than the spacing "hν" between energy levels, the exponential argument "βhν" is much less than one and the average energy becomes "kBT", in agreement with the equipartition theorem (Figure 10). However, at low temperatures, when "hν" >> "kBT", the average energy goes to zero — the higher-frequency energy levels are "frozen out" (Figure 10). As another example, the internal excited electronic states of a hydrogen atom do not contribute to its specific heat as a gas at room temperature, since the thermal energy "kBT" (roughly 0.025 eV) is much smaller than the spacing between the lowest and next higher electronic energy levels (roughly 10 eV). Similar considerations apply whenever the energy level spacing is much larger than the thermal energy. For example, this reasoning was used by Other, more subtle quantum effects can lead to corrections to equipartition, such as ee also * Notes and references Further reading * * * * * * * * ASIN B00085D6OO * External links * [http://webphysics.davidson.edu/physlet_resources/thermo_paper/thermo/examples/ex20_4.html Applet demonstrating equipartition in real time for a mixture of monatomic and diatomic gases]
Wikimedia Foundation.
2010.
.gif|frame|right|Figure_9._Energy_is_)
*
*
*
* [http://www.sciencebits.com/StellarEquipartition The equipartition theorem in stellar physics] , written by Nir J. Shaviv, an associate professor at the Racah Institute of Physics in the