- Dehydroalanine
-
Dehydroalanine 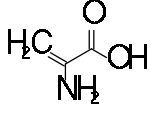
 2-Aminoprop-2-enoic acid
2-Aminoprop-2-enoic acidIdentifiers CAS number 1948-56-7 PubChem 123991 ChemSpider 110510 
DrugBank DB02688 KEGG C02218 
ChEBI CHEBI:17123 
Jmol-3D images Image 1
Image 2- C=C(C(=O)O)N
O=C(O)C(=C)N
Properties Molecular formula C3H5NO2 Molar mass 87.08 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dehydroalanine (or (alpha)-(beta)-di-dehydroalanine) is an uncommon amino acid found in peptides of microbial origin (an unsaturated amino acid).
Dehydroalanine (DHA) is also found in food proteins, including casein, that have been heated and/or treated with an alkali such as sodium hydroxide (NaOH). These treatments can dehydrate serine in the protein chain. In food, DHA frequently alkylates lysine to yield the crosslinking aminoacid lysinoalanine (LAL). Lysinoalanine, N6-(DL-2-amino-2-carboxyethyl)-L-lysine, an unusual amino acid implicated as a renal toxic factor in laboratory rats (kidney damage), has been found in proteins of home-cooked and commercial foods and ingredients. Although it had been reported to occur in both edible and non-food proteins only after alkali treatment (such as occurs in soybean processing), it has also been identified in food proteins that had not been subjected to alkali. Lysinoalanine can also be generated in a variety of proteins when heated under non-alkaline conditions.
Many dehydroalanine-containing peptides are toxic or antibiotic and constitute parts of lantibiotics or microcystins.
See also
References

This article about an amine is a stub. You can help Wikipedia by expanding it. - C=C(C(=O)O)N
