- Sodium percarbonate
-
Sodium percarbonate 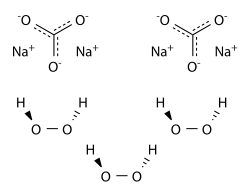
 sodium carbonate—hydrogen peroxide (2/3)Other namesPCS, solid hydrogen peroxide, Sodium carbonate hydrogen peroxide, sodium carbonate peroxyhydrate
sodium carbonate—hydrogen peroxide (2/3)Other namesPCS, solid hydrogen peroxide, Sodium carbonate hydrogen peroxide, sodium carbonate peroxyhydrateIdentifiers CAS number 15630-89-4 
PubChem 159762 ChemSpider 13399092 
EC number 239-707-6 Jmol-3D images Image 1 - [Na+].[O-]C(=O)OO
Properties Molecular formula Na2CO3·1.5H2O2 Molar mass 157.01 g/mol Appearance white solid Solubility in water 150 g/l Hazards EU Index Not listed Main hazards Irritant, Oxidizer Flash point Non-flammable Related compounds Other anions Sodium carbonate
Sodium bicarbonateRelated compounds Sodium perborate
Sodium persulfate
Sodium perphosphate (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium percarbonate is a chemical, an adduct of sodium carbonate and hydrogen peroxide (a perhydrate), with formula Na2CO3 · 1.5H2O2. It is a colorless, crystalline, hygroscopic and water-soluble solid.[1] It is used in some eco-friendly cleaning products and as a laboratory source of anhydrous hydrogen peroxide.
This product contains the carbonate anion, and should not be confused with sodium peroxocarbonate Na2CO4 or peroxodicarbonate Na2C2O6, which contain different anions.
Contents
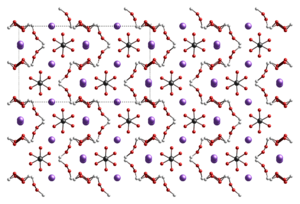
Structure
At room temperature, solid sodium percarbonate has the orthorhombic crystal structure, with the Cmca crystallographic space group. The structure changes to Pbca as the crystals are cooled below about −30 °C.[2]
Production
Sodium percarbonate is produced industrially by reaction of sodium carbonate and hydrogen peroxide, followed by crystallization. Also, dry sodium carbonate may be reacted directly with concentrated hydrogen peroxide solution. World production capacity of this compound was estimated at several hundred thousand tonnes for 2004.[3] It can be obtained in the laboratory by reacting the two substances in aqueous solution with proper control of the pH[4] or concentrations.[2]
Uses
Sodium percarbonate is an oxidizing agent and ingredient in a number of home and laundry cleaning products, including bleach products such as OxiClean and Tide laundry detergent.[1] It contains no phosphorus or nitrogen. Dissolved in water, it yields a mixture of hydrogen peroxide (which eventually decomposes to water and oxygen) and sodium carbonate ("soda ash").[1]
Sodium percarbonate can be used in organic synthesis as a convenient source of anhydrous H2O2, in particular in solvents that cannot dissolve the carbonate but can leach the H2O2 out of it.[5]
References
- ^ a b c Craig W. Jones (1999). Applications of hydrogen peroxide and its derivatives. Royal Society of Chemistry. ISBN 0854045368.
- ^ a b R. G. Pritchard and E. Islam (2003). "Sodium percarbonate between 293 and 100 K". Acta Crystallographica Section B B59: 596–605. doi:10.1107/S0108768103012291.
- ^ Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort (2005), "Peroxo Compounds, Inorganic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a19_177.pub2
- ^ J. M. Adams and R. G. Pritchard (1977). "The crystal structure of sodium percarbonate: an unusual layered solid". Acta Crystallographica Section B B33: 3650–3653. doi:10.1107/S0567740877011790.
- ^ McKillop, A (1995). "Sodium perborate and sodium percarbonate: Cheap, safe and versatile oxidising agents for organic synthesis". Tetrahedron 51: 6145. doi:10.1016/0040-4020(95)00304-Q.
External links
- Organic Chemistry Portal: Sodium percarbonate
- Record in the Household Products Database of NLM
Categories:- Sodium compounds
- Peroxides
- Carbonates
- Cleaning product components
- Antiseptics
- Bleaches
- Oxidizing agents
Wikimedia Foundation. 2010.
