- Cerulenin
-
Cerulenin 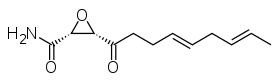 (2R,3S)-3-[(4E,7E)-nona-4,7-dienoyl]oxirane-2-carboxamide
(2R,3S)-3-[(4E,7E)-nona-4,7-dienoyl]oxirane-2-carboxamideIdentifiers CAS number 17397-89-6 
PubChem 5282054 ChemSpider 4445281 
DrugBank DB01034 KEGG C12058 
ChEBI CHEBI:171741 
ChEMBL CHEMBL45627 
Jmol-3D images Image 1 - O=C(CC/C=C/C/C=C/C)[C@H]1O[C@H]1C(=O)N
- InChI=1S/C12H17NO3/c1-2-3-4-5-6-7-8-9(14)10-11(16-10)12(13)15/h2-3,5-6,10-11H,4,7-8H2,1H3,(H2,13,15)/b3-2+,6-5+/t10-,11-/m1/s1

Key: GVEZIHKRYBHEFX-NQQPLRFYSA-N
InChI=1/C12H17NO3/c1-2-3-4-5-6-7-8-9(14)10-11(16-10)12(13)15/h2-3,5-6,10-11H,4,7-8H2,1H3,(H2,13,15)/b3-2+,6-5+/t10-,11-/m1/s1
Key: GVEZIHKRYBHEFX-NQQPLRFYBP
Properties Molecular formula C12H17NO3 Molar mass 223.27 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cerulenin is an antifungal antibiotic that inhibits fatty acid and steroid biosynthesis. In fatty acid synthesis, it has been reported to bind in equimolar ratio to b-keto-acyl-ACP synthase, one of the seven moieties of fatty acid synthase, blocking the interaction of malonyl-CoA. It also has the related activity of stimulating fatty acid oxidation through the activation of CPT1, another enzyme normally inhibited by malonyl-CoA. Inhibition involves covalent thioacylation that permanently inactivates the enzymes.[1] These two behaviors may increase the availability of energy in the form of ATP, perhaps sensed by AMPK, in the hypothalamus.[2]
In sterol synthesis, cerulenin inhibits HMG-CoA synthetase activity.[3] It was also reported that cerulenin specifically inhibited fatty acid biosynthesis in Saccharomyces cerevisiae without having an effect on sterol formation.[3]. But in general conclusion, cerulenin has inhibitory effects on sterol synthesis.
Cerulenin causes a dose-dependent decrease in HER2/neu protein levels in breast cancer cells, from 14% at 1.25 to 78% at 10 milligrams per liter, and targeting of fatty acid synthase by related drugs has been suggested as a possible treatment.[4] Antiproliferative and pro-apoptotic effects have been shown in colon cells as well.[5] At an intraperitoneal dose of 30 milligrams per kilogram, it has been shown to inhibit feeding and induce dramatic weight loss in mice by a mechanism similar to, but independent or downstream of, leptin signaling.[6] It is found naturally in the industrial strain Cephalosporium caerulens (Sarocladium oryzae, the sheath rot pathogen of rice).
External links
References
- ^ Straub SG, Yajima H, Komatsu M, Aizawa T, Sharp GW (February 2002). "The effects of cerulenin, an inhibitor of protein acylation, on the two phases of glucose-stimulated insulin secretion". Diabetes 51 Suppl 1 (90001): S91–5. doi:10.2337/diabetes.51.2007.S91. PMID 11815464. http://diabetes.diabetesjournals.org/cgi/content/full/51/suppl_1/S91.
- ^ Reviewed in Ronnett GV, Kleman AM, Kim EK, Landree LE, Tu Y (August 2006). "Fatty acid metabolism, the central nervous system, and feeding". Obesity (Silver Spring) 14 Suppl 5: 201S–207S. doi:10.1038/oby.2006.309. PMID 17021367.
- ^ a b Ohno T, Awaya J, Kesado T, Nomura S, Omura S (October 1974). "Mechanism of Action of CM-55, a Synthetic Analogue of the Antilipogenic Antibiotic Cerulenin". Antimicrob. Agents Chemother. 6 (4): 387–92. PMC 444657. PMID 4157441. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=4157441.
- ^ Menendez JA, Vellon L, Mehmi I, et al. (July 2004). "Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells". Proc. Natl. Acad. Sci. U.S.A. 101 (29): 10715–20. doi:10.1073/pnas.0403390101. PMC 490000. PMID 15235125. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=490000.
- ^ Huang P, Zhu S, Lu S, Dai Z, Jin Y (April 2000). "[An experimental study on cerulenin induced apoptosis of human colonic cancer cells]" (in Chinese). Zhonghua Bing Li Xue Za Zhi 29 (2): 115–8. PMID 11866903.
- ^ Ghosh MK, Amudha R, Jayachandran S, Sakthivel N (2002). "Detection and quantification of phytotoxic metabolites of Sarocladium oryzae in sheath rot-infected grains of rice". Lett. Appl. Microbiol. 34 (6): 398–401. doi:10.1046/j.1472-765X.2002.01111.x. PMID 12028418.
Categories:- Antifungals
- Amides
- Ketones
- Alkenes
- Epoxides
Wikimedia Foundation. 2010.
