- Magellanine
-
Magellanine 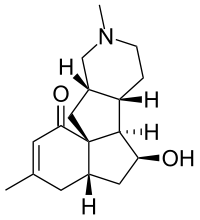 (4aS,6S,6aR,6bS,10aS,11aS)-6-Hydroxy-3,9-dimethyl-4,4a,5,6,6a,6b,7,8,9,10,10a,11-dodecahydro-1H-benzo[3a,4]pentaleno[2,1-c]pyridin-1-one
(4aS,6S,6aR,6bS,10aS,11aS)-6-Hydroxy-3,9-dimethyl-4,4a,5,6,6a,6b,7,8,9,10,10a,11-dodecahydro-1H-benzo[3a,4]pentaleno[2,1-c]pyridin-1-oneIdentifiers CAS number 61273-75-4 
PubChem 442489 ChemSpider 390919 
Jmol-3D images Image 1 - O=C1/C=C(/C)C[C@@H]2[C@@]14[C@H]([C@@H](O)C2)[C@@H]3[C@@H](CN(C)CC3)C4
- InChI=1S/C17H25NO2/c1-10-5-12-7-14(19)16-13-3-4-18(2)9-11(13)8-17(12,16)15(20)6-10/h6,11-14,16,19H,3-5,7-9H2,1-2H3/t11-,12+,13+,14+,16+,17-/m1/s1

Key: ADRPSBGLUHNVOU-INSRFAMQSA-N
InChI=1/C17H25NO2/c1-10-5-12-7-14(19)16-13-3-4-18(2)9-11(13)8-17(12,16)15(20)6-10/h6,11-14,16,19H,3-5,7-9H2,1-2H3/t11-,12+,13+,14+,16+,17-/m1/s1
Key: ADRPSBGLUHNVOU-INSRFAMQBF
Properties Molecular formula C17H25NO2 Molar mass 275.39 g mol−1 Melting point 165-166 °C[1]
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references (-)-Magellanine is a member of the Lycopodium alkaloid class of natural products. It was isolated from the club moss Lycopodium magellanicum in 1976.[1] It has been synthesized five times, with the first synthesis having been completed by the Larry E. Overman group at the University of California, Irvine in 1993.[2] It has also been synthesized by the Leo Paquette group in 1993 at The Ohio State University,[3] the Chun-Chen Liao group in 2002 at National Tsing Hua University,[4] the Miyuki Ishikazi and Tamiko Takahashi groups in 2005 at the Josai International University and Tokyo University of Science,[5] and the Chisato Mukai group in 2007 at the Kanazawa University.[6] One partial synthesis was completed by the A. I. Meyers group in 1995 at Colorado State University. [7]
Biosynthetically, it is thought to have been derived from lysine. This was determined by conducting feeding studies of radiolabeled precursors. [8]
References
- ^ a b Isolation and Structure Canadian Journal of Chemistry, 1976, 54:(18) 2893-2899.
- ^ Larry E. Overman Synthesis. J. Am. Chem. Soc., 1993, 115 (7), pp 2992–2993.
- ^ Leo Paquette Synthesis J. Am. Chem. Soc., 1994, 116 (11), pp 4689–4696.
- ^ Chun-Chen Liao Synthesis Angew. Chem. Int. Ed. 2002, 41, No. 21, 4090-4093.
- ^ Miyuki Ishikazi and Yamiko Takahashi Synthesis Tetrahedron 61 (2005) 4053–4065.
- ^ Chisato Mukai Synthesis J. Org. Chem. 2007, 26, 10147-10154.
- ^ A. I. Meyers Partial Synthesis J. Chem. Soc., Chem. Commun., 1995, 2511-2512.
- ^ Biosynthesis of the Lycopodium Alkaloids Nat. Prod. Rep., 2004, 21, 752-772.
Categories:- Alkaloids
Wikimedia Foundation. 2010.
