- Thianthrene
-
Thianthrene 
 ThianthreneOther namesThianthren; 9,10-Dithiaanthracene; Di-o-phenylene disulfide
ThianthreneOther namesThianthren; 9,10-Dithiaanthracene; Di-o-phenylene disulfideIdentifiers CAS number 92-85-3 
PubChem 7109 ChemSpider 6842 
EC number 202-197-0 ChEMBL CHEMBL488176 
Jmol-3D images Image 1 - S1c3c(Sc2c1cccc2)cccc3
Properties[1] Molecular formula C12H8S2 Molar mass 216.32 g mol−1 Melting point 151-155 °C, 424-428 K, 304-311 °F
Boiling point 364-366 °C, 637-639 K, 687-691 °F
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
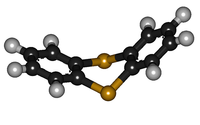
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Thianthrene is a sulfur-containing heterocyclic chemical compound. It is the sulfur analog of dibenzodioxin (commonly known as dioxin). Unlike dioxins, the shape of thianthrene is not planar. It is bent, with an angle of 128° between the two benzene rings.[2][3][4]
Thianthrene can be prepared by reacting disulfur dichloride with benzene in the presence of aluminium chloride.[5]
History
Thianthrene was first synthesized by John Stenhouse by dry distillation of benzenesulfonic acid sodium salt.[6] Thianthrene reacts with sulfuric acid forming a red product. R. Wizinger was the first to publish the structure of the colour giving cationic radical in 1929.[7] Although the structure was published several researchers used Thianthrene•+ for Electron paramagnetic resonance studies in the 1960s. Four different publications on the structure of Thianthrene•+ were published without knowledge of the other research.[8]
References
- ^ Thianthrene at Sigma-Aldrich
- ^ Hosoya, S. (1963). "Molecular shapes of thianthrene and related heterocyclic compounds". Acta Crystallographica 16 (4): 310–312. doi:10.1107/S0365110X63000797.
- ^ Gallaher, K. L.; Bauer, S. H. (1975). "Structure and inversion potential of thianthren". Journal of the Chemical Society, Faraday Transactions 2 71: 1173–1182. doi:10.1039/F29757101173.
- ^ Aroney, M. J.; Le Fèvre, R. J. W.; Saxby, J. D. (1965). "92. Molecular polarisability. The apparent conformations of thianthren and of three of its oxides as solutes in benzene". Journal of the Chemical Society (Resumed): 571–575. doi:10.1039/JR9650000571.
- ^ US patent 3997560, "Process for the manufacture of thianthrene", issued 1976-12-14.
- ^ Stenhouse, J. (1869). "Ueber die Producte der trockenen Destillation der sulfobenzolsauren Salze". Annalen der Chemie und Pharmacie 149 (2): 247–255. doi:10.1002/jlac.18691490216.
- ^ W. Dilthey: Versammlungsberichte Bonner Chemische Gesellschaft, Angewandte Chemie, Volume 42, Issue 24, pp. 668–670, 15. June 1929; doi:10.1002/ange.19290422405.
- ^ Shine, Henry J. (1998-07). "EPR and the History of the Thianthrene Cation Radical". Foundations of modern EPR. ISBN 9789810232955. http://books.google.com/?id=1LdKSYIQtPYC&pg=PA202.
Categories:- Thianthrenes
Wikimedia Foundation. 2010.
