- Diphosphane
-
This article is about P2H4. For other uses, see diphosphines.
Diphosphane 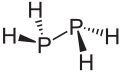 DiphosphaneSystematic nameDiphosphane (substitutive)
DiphosphaneSystematic nameDiphosphane (substitutive)
Tetrahydidodiphosphorus(P—P) (additive)Other namesDiphosphineIdentifiers CAS number 13445-50-6 
PubChem 139283 ChemSpider 122832 
ChEBI CHEBI:35880 
Jmol-3D images Image 1 - PP
Properties Molecular formula H4P2 Molar mass 65.98 g mol−1 Exact mass 65.978823152 g mol-1 Melting point -99 °C, 174 K, -146 °F
Boiling point 52 °C, 325 K, 126 °F
Related compounds Related compounds Hydrazine  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diphosphane is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air. An older name is diphosphine.
Contents
Properties, preparation, reactions
Diphosphane adopts the gauche conformation (like hydrazine, less symmetrical than shown in the image) with a P-P distance of 2.219 angstroms. It is nonbasic, unstable at room temperature, and spontaneously flammable in air. It is only poorly soluble in water but dissolves in organic solvents. Its 1H NMR spectrum consists of 32 lines resulting from an A2XX'A'2 splitting system.[1]
Diphosphane is produced by the hydrolysis of calcium monophosphide, which can be described as the Ca2+ derivative of P24-. According to an optimized procedure, hydrolysis of 400 g of CaP at -30 °C gives about 20 g of product, slightly contaminated with phosphine.
Reaction of diphosphine with butyl lithium affords a variety of condensed polyphosphine compounds.
Organic diphosphanes
A variety of organic derivatives of diphosphane are known. These species are prepared by reductive coupling, e.g. from chlorodiphenylphosphine:
- ClPPh2 + 2 Na → Ph2PPPh2 + 2 NaCl
The methyl compound P2Me4 is prepared by the reaction of thiophosphoryl chloride with methylmagnesium bromide.
See also
References
- ^ Marianne Baudler, Klaus Glinka (1993). "Monocyclic and polycyclic phosphines". Chem. Rev. 93 (4): 1623–1667. doi:10.1021/cr00020a010.
Categories:- Inorganic compound stubs
- Phosphorus hydrides
Wikimedia Foundation. 2010.
