- 4,4'-Dihydroxybenzophenone
-
4,4'-Dihydroxybenzophenone 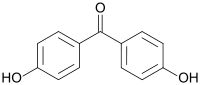 Other namesBenzophenone, 4,4’dihydroxy-(7Cl,8Cl); 4,4’-dihydroxydiphenyl ketone; Bis(4-hydroxyphenyl) ketone; HBP; HBP (ketone); NSC
Other namesBenzophenone, 4,4’dihydroxy-(7Cl,8Cl); 4,4’-dihydroxydiphenyl ketone; Bis(4-hydroxyphenyl) ketone; HBP; HBP (ketone); NSCIdentifiers CAS number 611-99-4 ChemSpider 62365 
DrugBank DB07635 KEGG C14220 
ChEMBL CHEMBL194859 
Jmol-3D images Image 1 - O=C(c1ccc(O)cc1)c2ccc(O)cc2
Properties Molecular formula C13H10O3 Molar mass 214.22 g/mol Appearance Off white/yellow solid Density 1.302g/cm3 Melting point 213-215 °C
Boiling point 444.8 °C @760mmHg
Solubility in water 0.45 g/L Hazards MSDS MSDS by Fisher Scientific Flash point 237 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 4,4'-Dihydroxybenzophenone is an organic compound with the formula (HOC6H4)2CO. This off-white solid is a precursor to or a degradation product of diverse commercial materials. It is a potential endocrine disruptor.[1]
Synthesis
4,4'-Dihydroxybenzophenone is prepared by the rearrangement of p-hydroxyphenylbenzoate:
- HOC6H4CO2C6H5 → (HOC6H4)2CO
Alternatively, p-hydroxybenzoic acid can be converted to p-acetoxybenzoyl chloride. This acid chloride reacts with phenol to give, after deacetylation, 4,4'-dihydroxybenzophenone.
Uses
The main application of 4,4'-dihydroxybenzophenone is as a UV light stabilizer. It and its derivatives are found in cosmetics, plastics, films, adhesives and coatings, optical fiber, and printed circuit boards. It is the precursor to certain polycarbonate polymers.[2]
References
- ^ Eddine, Ali Nasser; von Kries, Jens P.; Podust, Mikhail V.; Warrier, Thulasi; Kaufmann, Stefan H. E.; Podust, Larissa M. "X-ray Structure of 4 , 4 '- Dihydroxybenzophenone Mimicking Sterol Substrate in the Active Site of Sterol 14a -Demethylase (CYP51)" Journal of Biological Chemistry (2008), 283, pp. 15152-15159. doi:10.1074/jbc.M801145200
- ^ David Parker, Jan Bussink, Hendrik T. van de Grampe, Gary W. Wheatley, Ernst-Ulrich Dorf, Edgar Ostlinning, Klaus Reinking "Polymers, High-Temperature" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002.doi:10.1002/14356007.a21_449
Categories:- Aromatic ketones
- Synthetic phenols
Wikimedia Foundation. 2010.
