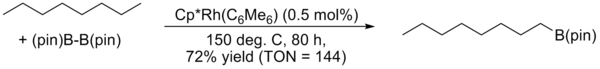- Carbon–hydrogen bond activation
-
Carbon–hydrogen bond activation or C−H activation may be defined as a reaction that cleaves a carbon–hydrogen bond. Often the term is restricted to reactions involving organometallic complexes and proceeding by coordination of a hydrocarbon to the inner-sphere of metal, either via an intermediate “alkane or arene complex” or as a transition state leading to a "M−C" intermediate.[1][2][3] Important to this definition is the requirement that during the C−H cleavage event the hydrocarbyl species remains associated in the inner-sphere and under the influence of “M”.
Theoretical studies as well as experimental investigations indicate that C−H bonds, which are traditionally considered unreactive, can be cleaved by coordination. Much research effort has been devoted to the design and synthesis of new reagents and catalysts that can affect CH activation. A significant driver for this type of research is the prospect that C−H activation could enable the conversion of cheap and abundant alkanes into valuable functionalized organic compounds.
Contents
Historic overview
The first C−H activation reaction is often attributed to Otto Dimroth who in 1902 reported that benzene with mercury(II) acetate (See: organomercury), but some scholars do not view this reaction as being truly C−H activation. As observed Goldman & Goldberg[1] C−H activation resembles aspects of H−H activation: both can be achieved by electrophilic or oxidative addition.
The first true C−H activation reaction was reported by Joseph Chatt in 1965 [4] with insertion of a ruthenium atom ligated to dmpe in the C−H bond of naphthalene. In 1969 A.E. Shilov reported that potassium tetrachloroplatinate induced isotope scrambling between methane and heavy water. The pathway was proposed to involve binding of methane to Pt(II). In 1972 the Shilov group was able to produce methanol and methyl chloride in a similar reaction of stoichiometric potassium tetrachloroplatinate, catalytic potassium hexachloroplatinate, methane and water. As Shilov worked and published in Cold War Soviet Union his work was largely ignored by Western scientists. This so-called Shilov system is today one of the few true catalytic systems for mild alkane functionalizations.[1]
On the other side of the spectrum, oxidative addition, M. L. H. Green in 1970 reported on the photochemical insertion of tungsten (as a Cp2WH2 complex) in a benzene C−H bond [5] and George M. Whitesides in 1979 was the first to carry out an intramolecular aliphatic C−H activation [6]
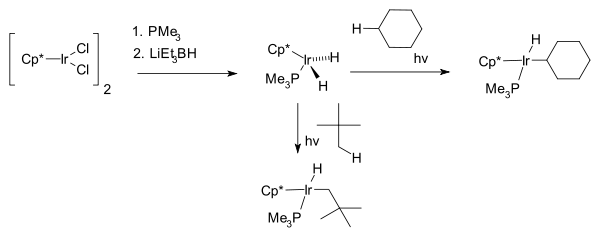
The next breakthrough was reported independently by two research groups 1982, by R. G. Bergman with the first photochemical C−H activation of completely saturated hydrocarbons cyclohexane and neopentane forming the hydridoalkylmetal complex Cp*Ir(PMe3)H(C6H5) where Cp* is a pentamethylcyclopentadienyl ligand.[7]
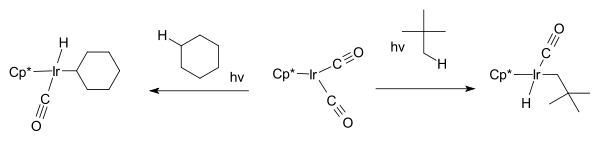
W.A.G. Graham found that the same hydrocarbons react with Cp*Ir(CO)2 to afford iridium hydrido complexes.[8]
The first part of the reaction; the iridium complex reacting with the cycloalkane is an example of oxidative addition where the hydrogen is removed from the alkane and oxidatively added to the metal.
J.F. Hartwig reported a highly regioselective arene and alkane borylation catalyzed by a rhodium complex in 1999 and 2000. In the case of alkanes, exclusive terminal functionalization was observed.[9]
Scope
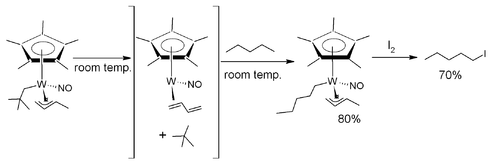
In one study[10] the alkane pentane is selectively converted to the halocarbon 1-iodopentane with the aid of a tungsten complex.
The tungsten complex is fitted with a pentamethylcyclopentadienyl, a nitrosyl, a 3η 1-butene and a neopentanyl CH2C(CH3)3 ligand. It is thermally unstable and when dissolved in pentane at room temperature it loses neopentane (gains a proton) and coordinates with a pentane ligand (loses a proton). This proton exchange proceeds via a 16 electron intermediate with a butadiene ligand after beta elimination. In a separate step iodine is added at −60°C and 1-iodopentane is released.
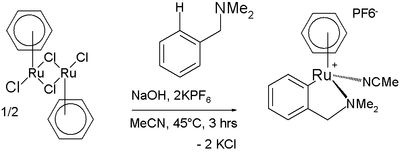
Arene C−H bonds can also be activated by metal complexes despite being fairly unreactive. One manifestation is found in the Murai olefin coupling.[11] In one reaction a ruthenium complex reacts with N,N-dimethylbenzylamine in a cyclometalation also involving C−H activation [12]:
An alkene C−H bond activation with a rhodium catalyst is demonstrated in the synthesis of this strained bicyclic enamine [13]:
Reaction conditions
Most C-H bond activations proceed under rather harsh reaction conditions (high T, strongly acidic or basic conditions, strong oxidant), significantly lowering their attractiveness. However, more and more quite mild reactions have been developed, significantly expanding the scope of these exciting transformations.[14]
See also
References
- ^ a b c Organometallic C−H Bond Activation: An Introduction Alan S. Goldman and Karen I. Goldberg ACS Symposium Series 885, Activation and Functionalization of C−H Bonds, 2004, 1–43
- ^ Arndtsen, B. A.; Bergman, R. G.; Mobley, T. A.; Peterson, T. H. “Selective Intermolecular Carbon–Hydrogen Bond Activation by Synthetic Metal Complexes in Homogeneous Solution.” Accounts of Chemical Research, 1995: 28 (3) 154–162.
- ^ Periana, R. A.; Bhalla, G.; Tenn, W. J., III, Young, K. J. H.; Liu, X. Y.; Mironov, O.; Jones, C.; Ziatdinov, V. R. “Perspectives on some challenges and approaches for developing the next generation of selective, low temperature, oxidation catalysts for alkane hydroxylation based on the C−H activation reaction.” Journal of Molecular Catalysis A: Chemical, 2004: 220 (1) 7–25. doi:10.1016/j.molcata.2004.05.036
- ^ The tautomerism of arene and ditertiary phosphine complexes of ruthenium(0), and the preparation of new types of hydrido-complexes of ruthenium(II) J. Chatt and J. M. Davidson, J. Chem. Soc. 1965, 843 doi:10.1039/JR9650000843
- ^ Formation of a tangsten phenyl hydride derivatives from benzene M. L. Green, P. J. Knowles, J. Chem. Soc. D, 1970, (24),1677–1677 doi:10.1039/C29700001677
- ^ Thermal generation of bis(triethylphosphine)-3,3-dimethylplatinacyclobutane from dineopentylbis(triethylphosphine)platinum(II) Paul Foley, George M. Whitesides J. Am. Chem. Soc. 1979; 101(10); 2732–2733. doi:10.1021/ja00504a041
- ^ Carbon–hydrogen activation in saturated hydrocarbons: direct observation of M + R−H → M(R)(H) Andrew H. Janowicz, Robert G. Bergman J. Am. Chem. Soc.; 1982; 104(1); 352–354.doi:10.1021/ja00365a091
- ^ Oxidative addition of the carbon–hydrogen bonds of neopentane and cyclohexane to a photochemically generated iridium(I) complex James K. Hoyano, William A. G. Graham J. Am. Chem. Soc. 1982; 104(13); 3723–3725. doi:10.1021/ja00377a032
- ^ Thermal, Catalytic, Regiospecific Functionalization of Alkanes Huiyuan Chen, Sabine Schlecht, Thomas C. Semple, John F. Hartwig Science 2000; 287(5460); 1995–1997. doi:10.1126/science.287.5460.1995
- ^ Selective Activation and Functionalization of Linear Alkanes Initiated under Ambient Conditions by a Tungsten Allyl Nitrosyl Complex Jenkins Y. K. Tsang, Miriam S. A. Buschhaus, and Peter Legzdins J. Am. Chem. Soc.; 2007; 129(17) pp 5372–5373; (Communication) doi:10.1021/ja0713633
- ^ Murai, Shinji; Kakiuchi, Fumitoshi; Sekine, Shinya; Tanaka, Yasuo; Kamatani, Asayuki; Sonoda, Motohiro; Chatani, Naoto (1993). "Efficient catalytic addition of aromatic carbon–hydrogen bonds to olefins". Nature 366 (6455): 529–531. doi:10.1038/366529a0.
- ^ Formation of a Ruthenium–Arene Complex, Cyclometallation with a Substituted Benzylamine, and Insertion of an Alkyne Chetcuti, Michael J.; Ritleng, Vincent. J. Chem. Educ. 2007, 84, 1014. Abstract
- ^ The Stereoselective Formation of Bicyclic Enamines with Bridgehead Unsaturation via Tandem C−H Bond Activation/Alkenylation/ Electrocyclization Sirilata Yotphan, Robert G. Bergman, and Jonathan A. Ellman J. AM. CHEM. SOC. 2008, 130, 2452–2453 doi:10.1021/ja710981b
- ^ Towards Mild Metal-Catalyzed C-H Bond Activation J. Wencel-Delord, T. Dröge, F. Liu, F. Glorius Chem. Soc. Rev. 2011, 40, 4740-4761 doi:10.1039/C1CS15083A
Further reading
- “Activation of C−H Bonds by Metal Complexes”, A. E. Shilov, G. B. Shul’pin, Chem. Rev. 1997, 97, 2879–2932. doi:10.1021/cr9411886
- “Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes”, A. E. Shilov, G. B. Shul’pin, Kluwer Academic Publishers, Dordrecht/Boston/London, 2000 (552 p) (Springer, ISBN 978-0-7923-6101-5). http://www.springer.com/chemistry/physical+chemistry/book/978-0-7923-6101-5
- “Alkane C−H activation and functionalization with homogeneous transition metal catalysts: a century of progress – a new millennium in prospect”, R. H. Crabtree, J. Chem. Soc., Dalton Trans. 2001, 17, 2437–2450. doi:10.1039/B103147N
- “Organometallic alkane CH activation”, R. H. Crabtree, J. Organometal. Chem. 2004, 689, 4083–4091. doi:10.1016/j.jorganchem.2004.07.034
- “Mechanistic Aspects of C−H Activation by Pt Complexes”, M. Lersch, M.Tilset, Chem. Rev. 2005, 105, 2471−2526. doi:10.1021/cr030710y
- “Recent Advances in the Platinum-mediated CH Bond Functionalization”, A. N. Vedernikov, Curr. Org. Chem. 2007, 11, 1401−1416.
- “Catalytic C−H functionalization by metalcarbenoid and nitrenoid insertion”, H. M. L. Davies, J. R. Manning, Nature, 2008, 451, 417−424, doi:10.1038/nature06485
- "Mechanisms of C−H bond activation: rich synergy between computation and experiment”, Y. Boutadla, D. L. Davies, S. A. Macgregor, A. I. Poblador-Bahamonde, Dalton Trans. 2009, 5820−5831. doi:10.1039/B904967C
- “C−H Bond Activation in Transition Metal Species from a Computational Perspective”, D. Balcells, E. Clot, O. Eisenstein, Chem. Rev. 2010, 110, 749–823. doi:10.1021/cr900315k
- “Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions”, T. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147–1169. doi:10.1021/cr900184e
- “Selectivity enhancement in functionalization of C−H bonds: A review”, G. B. Shul’pin, Org. Biomol. Chem. 2010, 8, 4217–4228. doi:10.1039/c004223d
Categories:
Wikimedia Foundation. 2010.