- CZTS
-
CZTS 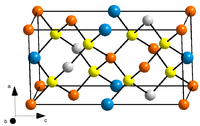
CZTS crystal structure. Orange: Cu, blue: Sn, grey: Zn/Fe, yellow: S.Other namescopper zinc tin sulfideIdentifiers CAS number 12158-89-3 Properties Molecular formula Cu2ZnSnS4 Molar mass 439.471 Appearance Greenish black crystals Density 4.56 g/cm3[3] Melting point 990 °C[4]
Band gap 1.4–1.5 eV[1][2] Structure Crystal structure Tetragonal[3] Lattice constant a = 0.5435 nm, c = 1.0843 nm, Z = 2 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references CZTS is an abbreviation for a quaternary compound of copper, zinc, tin and sulfur with the chemical formula Cu2ZnSnS4. CZTS occurs in nature as the mineral kesterite. This material is a semiconductor and is closely related to copper indium gallium selenide (CIGS, Cu(In,Ga)Se2). CZTS may contain some iron which substitutes for zinc, and selenium which replaces sulfur; when most sulfur atoms are substituted by selenium, the abbreviation is changed to CZTSe. Thin films of CZTS and CIGS are used in solar cells.
CZTS in solar cells
After the photovoltaic effect was discovered in CZTS in 1988[5] this material was considered as an alternative to CIGS for commercial solar cell systems. The advantage of CZTS is the lack of the relatively rare and expensive element indium. The British Geological Survey Risk List 2011 gave indium a "relative supply risk index" of 6.5, where the maximum was 8.5.[6]
In 2010, a solar energy conversion efficiency of about 10% was achieved in a CZTS device.[7] CZTS technology is now being developed by several private companies.[8]
References
- ^ Ichimura, Masaya; Nakashima, Yuki (2009). "Analysis of Atomic and Electronic Structures of Cu2ZnSnS4Based on First-Principle Calculation". Japanese Journal of Applied Physics 48 (9): 090202. doi:10.1143/JJAP.48.090202.
- ^ Katagiri, Hironori; Saitoh, Kotoe; Washio, Tsukasa; Shinohara, Hiroyuki; Kurumadani, Tomomi; Miyajima, Shinsuke (2001). "Development of thin film solar cell based on Cu2ZnSnS4 thin films". Solar Energy Materials and Solar Cells 65: 141. doi:10.1016/S0927-0248(00)00088-X.
- ^ a b Guen, L.; Glaunsinger, W.S. (1980). "Electrical, magnetic, and EPR studies of the quaternary chalcogenides Cu2AIIBIVX4 prepared by iodine transport". Journal of Solid State Chemistry 35: 10. doi:10.1016/0022-4596(80)90457-0.
- ^ Matsushita, H.; Ichikawa, T.; Katsui, A. (2005). "Structural, thermodynamical and optical properties of Cu2-II-IV-VI4 quaternary compounds". Journal of Materials Science 40 (8): 2003. doi:10.1007/s10853-005-1223-5.
- ^ Ito, K.; Nakazawa, T. (1988). "Electrical and Optical Properties of Stannite-Type Quaternary Semiconductor Thin Films". Japanese Journal of Applied Physics 27: 2094. doi:10.1143/JJAP.27.2094.
- ^ Risk list 2011. A new supply risk index for chemical elements or element groups which are of economic value. Minerals UK
- ^ Todorov, T. K.; Reuter, K. B.; Mitzi, D. B. (2010). "High-Efficiency Solar Cell with Earth-Abundant Liquid-Processed Absorber". Advanced Materials 22 (20): E156. doi:10.1002/adma.200904155.
- ^ Solar Frontier and IBM Sign Agreement to Develop CZTS Solar Cell Technology. Solar Frontier. October 19, 2010
Further reading
- Jonathan J. Scragg (2011). Copper Zinc Tin Sulfide Thin Films for Photovoltaics: Synthesis and Characterisation by Electrochemical Methods. Springer. ISBN 978-3-642-22918-3.
Categories:- Chemistry stubs
- Sulfides
- Solar cells
Wikimedia Foundation. 2010.
