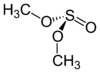
- Dimethyl sulfite
-
Dimethyl sulfite 
 MethoxysulfinyloxymethaneOther names
MethoxysulfinyloxymethaneOther namesIdentifiers CAS number 616-42-2 PubChem 69223 ChemSpider 62436 
EC number 210-481-0 ChEBI CHEBI:48858 
Jmol-3D images Image 1
Image 2- COS(=O)OC
O=S(OC)OC
Properties Molecular formula C2H6O3S Molar mass 110.088 g/mol Appearance Clear liquid Density 1.29 g/cm3 Boiling point 126 °C, 399 K, 259 °F
 sulfite (verify) (what is:
sulfite (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethyl sulfite is a sulfite ester with the chemical formula (CH3O)2SO.
Dimethyl sulfite is used as an additive in some polymers to prevent oxidation.[2] It is also a potentially useful high energy battery electrolyte solvent.[3]
Contents
Structure and conformation
The dimethyl sulfite molecule can adopt several conformations. The most stable is the GG conformer.[1] Each C–O bond is gauche to the S=O bond, depicted below.
Preparation
Although formally derived from sulfurous acid, dimethyl sulfite is prepared from thionyl chloride and methanol.
- OSCl2 + 2CH3OH → OS(OCH3)2 + 2HCl
See also
- Methyl methanesulfonate, a chemical with the same molecular formula but different arrangement of atoms
- Diethyl sulfite, a similar sulfite ester
- Dimethyl sulfoxide
- Dimethyl sulfate, a sulfate ester
References
- ^ a b Borba, A.; Gómez-Zavaglia, A.; Simões, P. N. N. L.; Fausto, R. (2005). "Matrix Isolation FTIR Spectroscopic and Theoretical Study of Dimethyl Sulfite". J. Phys. Chem. A 109 (16): 3578–3586. doi:10.1021/jp050020t.
- ^ Guenther, A.; Koenig, T.; Habicher, W. D.; Schwetlick, K. (1997). "Antioxidant action of organic sulfites. I. Esters of sulfurous acid as secondary antioxidants". Polymer Degradation and Stability 55 (2): 209–216. doi:10.1016/S0141-3910(96)00150-4.
- ^ N. P. Yao, E. D'Orsay, and D. N. Bennion (1968). "Behavior of Dimethyl Sulfite as a Potential Nonaqueous Battery Solvent". J. Electrochem. Soc. 115 (10): 999–1003. doi:10.1149/1.2410917.
External links
This article about an ester is a stub. You can help Wikipedia by expanding it. - COS(=O)OC