- Melamine cyanurate
-
Melamine-cyanuric acid complex[1] 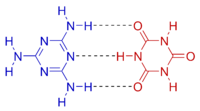 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, compd. with 1,3,5-triazine-2,4,6-triamine (1:1)Other namesMelamine-cyanuric acid compound, melamine-cyanuric acid adduct, melamine cyanurate, melamine isocyanurate
1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, compd. with 1,3,5-triazine-2,4,6-triamine (1:1)Other namesMelamine-cyanuric acid compound, melamine-cyanuric acid adduct, melamine cyanurate, melamine isocyanurateIdentifiers CAS number 37640-57-6, (Fmr. 70371-20-9) PubChem 93198 MeSH melamine+cyanurate Jmol-3D images Image 1 - n1c(N)nc(N)nc1N.N1C(=O)NC(=O)NC1=O
Properties Molecular formula C6H9N9O3 (C3H6N6·C3H3N3O3)
Molar mass 255.19 g/mol Solubility in water none  cyanurate (verify) (what is:
cyanurate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Melamine cyanurate, also known as melamine-cyanuric acid adduct or melamine-cyanuric acid complex, is a crystalline complex formed from a 1:1 mixture of melamine and cyanuric acid. The substance is not a salt despite its non-systematic name melamine cyanurate. The complex is held together by an extensive two-dimensional network of hydrogen bonds between the two compounds, reminiscent to that seen in DNA base pairing.[2] Melamine cyanurate forms spoke-like crystals from aqueous solutions [3] and has been implicated as a causative agent for toxicity seen in the Chinese protein export contamination and the 2007 pet food recall.[3]
Contents
Chemistry
The substance is best described as a melamine-cyanuric acid co-crystallate, complex, or non-covalent adduct. The two compounds do not form a salt as suggested by its non-systematic trivial name melamine cyanurate.
Melamine and cyanuric acid form a jigsaw puzzle-like two-dimensional hydrogen bonding network because of the complementarity of the two compounds, similar to DNA base pairing.
Uses
Melamine cyanurate is commonly used as a fire retardant.
Toxicity
It has been considered to be more toxic than either melamine or cyanuric acid alone.[4]
LD50 in rats and mice (ingested):
- 4.1 g/kg - Melamine cyanurate
- 6.0 g/kg - Melamine[clarification needed]
- 7.7 g/kg - Cyanuric acid
A toxicology study conducted after recent pet food recalls concluded that the combination of melamine and cyanuric acid in diet does lead to acute renal failure in cats.[5] A 2008 study produced similar experimental results in rats and characterized the melamine and cyanuric acid in contaminated pet food from the 2007 outbreak.[6]
See also
References
- ^ EPA: Substance :
- ^ Perdigão LM, Champness NR, Beton PH (2006). "Surface self-assembly of the cyanuric acid-melamine hydrogen bonded network". Chem. Commun. (5): 538–540. doi:10.1039/b514389f. PMID 16432575.
- ^ a b Lili He, Yang Liu, Mengshi Lin, Joseph Awika, David R. Ledoux, Hao Li, Azlin Mustapha, (2008). "A new approach to measure melamine, cyanuric acid, and melamine cyanurate using surface enhanced Raman spectroscopy coupled with gold nanosubstrates". Sens. & Instrumen. Food Qual. 2: 66–71. doi:10.1007/s11694-008-9038-0.
- ^ A.A. Babayan, A.V.Aleksandryan, "Toxicological characteristics of melamine cyanurate, melamine and cyanuric acid", Zhurnal Eksperimental'noi i Klinicheskoi Meditsiny, Vol.25, 345-9 (1985). Original article in Russian.
- ^ Puschner et al. (November 2007). Assessment of melamine and cyanuric acid toxicity in cats. Journal of Veterinary Diagnostic Investigation. Retrieved on 2007-11-16.
- ^ Dobson et al. (August 2008). "Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs.". Toxicological Sciences. http://toxsci.oxfordjournals.org/cgi/content/full/106/1/251. Retrieved 2009-08-13.
Categories:- Amines
- Triazines
Wikimedia Foundation. 2010.

