- 1,1-Bis(diphenylphosphino)methane
-
1,1-bis(diphenylphosphino)methane 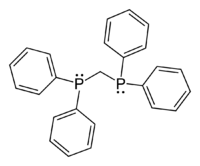
 methylenebis(diphenylphosphane)Other namesMethylenebis(diphenylphosphine)
methylenebis(diphenylphosphane)Other namesMethylenebis(diphenylphosphine)
dppmIdentifiers CAS number 2071-20-7 
ChemSpider 67509 
ChEMBL CHEMBL307780 
Jmol-3D images Image 1 - P(c1ccccc1)(c2ccccc2)CP(c3ccccc3)c4ccccc4
Properties Molecular formula C25H22P2 Molar mass 384.39 g/mol Appearance White powder or crystalline powder Melting point 118-122 °C
Solubility in water Insoluble in water Hazards MSDS External MSDS R-phrases 36/37/38 S-phrases 22-24/25  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand.[1] It is more specifically a chelating ligand because it is a ligand that is attached to the central metals (the phosphorus) by bonds from two or more donor atoms.
Synthesis and reactivity
1,1-Bis(diphenylphosphino)methane was first prepared by the reaction of sodium diphenylphosphide (Ph2PNa) with dichloromethane:[2]
- Ph3P + 2 Na → Ph2PNa + NaPh
- 2NaPPh2 + CH2Cl2 → Ph2PCH2PPh2 + 2 NaCl
The methylene group (CH2) in dppm (and especially its complexes) are mildly acidic. The ligand can be oxidized to give the corresponding oxides and sulfides CH2[P(E)Ph2]2 (E = O, S). The methylene group is even more acidic in these derivatives.
Coordination chemistry
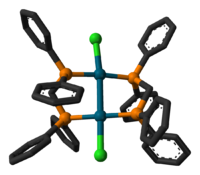
As a chelating ligand, 1,1-bis(diphenylphosphino)methane forms a four-membered ring with the constituents MP2C. The ligand promotes the formation of bimetallic complexes that feature five-membered M2P2C rings. In this way, dppm promotes the formation of bimetallic complexes. One such example is the dipalladium chloride, Pd2Cl2(dppm)2. In this complex, the oxidation state for the Pd centres are I. Bis(diphenylphosphino)methane gives rise to a family of coordination compounds known as A-frame complexes.[3]
References
- ^ Humphrey, Mark G.; Lee, Jeanne; Hockless, David C.R.; Skelton, Brian W.; & White, Allan H. (1993). "Mixed-Metal Cluster Chemistry". Organometallics 12 (3468): 3468. doi:10.1021/om00033a017.
- ^ W. Hewertson and H. R. Watson (1962). "The preparation of di- and tri-tertiary phosphines". J. Chem. Soc. 12: 1490–1494. doi:10.1039/JR9620001490.
- ^ Albéniz, Ana C. and Espinet, Pablo (2006). "Encyclopedia of Inorganic Chemistry". Encyclopedia of Inorganic Chemistry. doi:10.1002/0470862106.ia178. ISBN 0470860782.
- ^ G. Besenyei, L. Párkányia, E. Gács-Baitza, B. R. James (January 2002). "Crystallographic characterization of the palladium(I) dimers, syn-Pd2Cl2(dppmMe)2 and Pd2Cl2(dppm)2; solution conformational behavior of syn- and anti-Pd2Cl2(dppmMe)2 and their (μ-Se) adducts [dppmMe=μ-1,1-bis(diphenylphosphino)ethane, and DPPM=μ-bis(diphenylphosphino)methane]". Inorg. Chim. Acta 327 (1): 179–187. doi:10.1016/S0020-1693(01)00682-X.
Categories:- Bisphosphanes
Wikimedia Foundation. 2010.