- Diphenylphosphine
-
Diphenylphosphine 
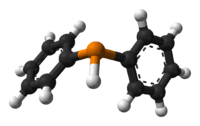 diphenylphosphane
diphenylphosphaneIdentifiers CAS number 829-85-6 
ChemSpider 63209 
Jmol-3D images Image 1 - c2c(Pc1ccccc1)cccc2
Properties Molecular formula C12H11P Molar mass 186.19 g/mol Appearance colorless liquid Density 1.07 g/cm3, liquid Boiling point 280 °C (553 K)
Solubility in water Insoluble Hazards MSDS External MSDS R-phrases 17-36/37/38 S-phrases 26-36  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diphenylphosphine, also known as diphenylphosphane, is organophosphorus compound with the formula (C6H5)2PH. This foul smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.
Contents
Synthesis
Diphenylphosphine can be prepared cheaply from triphenylphosphine.[1]
- PPh3 + 2 Li → LiPPh2 + LiPh
- LiPPh2 + H2O → Ph2PH + LiOH
Uses and reactions
In the laboratory, diphenylphosphine is a common intermediate. It readily deprotonated to give diphenylphosphide derivatives:[2]
- Ph2PH + nBuLi → Ph2PLi + nBuH
The preparation of phosphine ligands, Wittig-Horner reagents, and phosphonium salts are commonly accomplished by alkylating diphenylphosphine. The hydrogen atom connected to phosphorus undergoes Michael-like addition to activated alkenes, providing products with which to produce phosphine ligands such as 1,2-bis(diphenylphosphino)ethane (Ph2PC2H4PPh2). Diphenylphosphine and especially diphenylphosphide derivatives are nucleophiles, so they add to carbon – heteroatom double bonds.[2] For example, in the presence of concentrated hydrochloric acid at 100 °C, diphenylphosphine adds to the carbon atom in benzaldehyde to give (phenyl-(phenylmethyl)phosphoryl)benzene.
- Ph2PH + PhCHO → Ph2P(O)CH2Ph
Handling properties
During the handling of diphenylphosphine, care must be taken to avoid oxidation of diphenylphosphine.[3]
- Ph2PH + O2 → Ph2P(O)OH
The use of the diphenylphosphine–borane complex, Ph2PH•BH3 avoids the problem of phosphine oxidation by protecting the phosphine from oxidation and is available through chemical vendors.[2]
References
- ^ Wittenberg, D.; Gilman, H. (1958). "Lithium Cleavages of Triphenyl Derivatives of Some Group Vb Elements in Tetrahydrofuran". J. Org. Chem. 23 (7): 1063–1065. doi:10.1021/jo01101a613.
- ^ a b c Piotrowski, D. W. “Diphenylphosphine” Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons: New York, 2001. doi:10.1002/047084289X.rd427.
- ^ Svara, J., Weferling, N., Hofmann, T. “Organic Phosphorus Compounds” Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons: New York, 2006. doi:10.1002/14356007.a19_545.pub2.
Categories:- Aromatic compounds
- Phosphines
Wikimedia Foundation. 2010.
