- Thiophosphate
-
- For the thiophosphate esters see organothiophosphates.
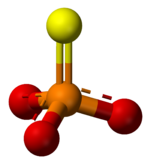
A thiophosphate (or phosphorothioate) is a family of compounds and anions with the general chemical formula PS4-xOx3- (x = 0, 1, 2, or 3). The state of protonation is usually not specified. They could be bound to as many as three protons for the neutral H3PS4-xOx species. Two protons correspond to the related monoanions, and one proton for the dianions. The trianions are highly basic and do not exist in appreciable concentrations in solution. Thiophosphates are tetrahedral anions, with the phosphorus at the center of the tetrahedron.
Contents
Monothiophosphate
Monothiophosphates are formally derived from the anion PO3S3-, which has C3v symmetry.. A number of insecticides feature organic monothiophosphate center, including diazinon, parathion, and malathion. They have the formula (RO)2PS(OAr), where R = methyl or ethyl and Ar = an aryl or related group. Coordination complexes of are known.[1]
In the laboratory monothiophosphate is used as an analogue of phosphate in biochemistry. Monothiophosphate esters are biochemical reagents used in the study transcription,[2] substitution interference assays.
Dithiophosphates
Dithiophosphates are formally derived from the anion PO2S23-, which has C2v symmetry. This species is the major product from the reaction of base (e.g., NaOH) with phosphorus pentasulfide. The sodium salt has the nominal formula Na3PO2S2, but is invariably hydrated. The main application of this dithiophosphate salt is in the purification of molybdenite ore. Organic thiophosphates feature the (RO)2PS2- anion (R = alkyl, aryl). Zinc dithiophosphate, e.g., Zn(S2P(OR)2)2]], are used as oil additives (R = alkyl).
Tri- and tetrathiophosphates
Trithiophosphates are formally derived from the anion POS33-, which has C3v symmetry. Tetrathiophosphate has the formula PS43-. Derivatives of these two anions are less commonly encountered.
References
- ^ Poat JC et al. (1990). "A thiophosphate bridged platinum–zinc hetero-bimetallic complex: [(Me2PhP)2Pt{OSP(OR)2}2ZnCl2". J. Chem. Soc., Chem. Commun.: 1036–1038. http://www.rsc.org/publishing/journals/article.asp?doi=C39900001036.
- ^ Lorsch JR, Bartel DP, Szostak JW. (1995). "Reverse transcriptase reads through a 2'–5' linkage and a 2'-thiphosphate in a template". Nucleic Acids Res. 23 (15): 2811–2814. doi:10.1093/nar/23.15.2811. PMC 307115. PMID 7544885. http://nar.oxfordjournals.org/cgi/content/abstract/23/15/2811.
Only P-O bonds P(III)Phosphite/OrganophosphiteP(V)With P-C bonds One P-C bondsP(III)P(V)Two P-C bondsP(III)P(V)Three P-C bondsP(III)P(V)With P-N bonds One P-N bondP(III)P(V)Phosphoramidate (e.g. Phosphocreatine)Two P-N bondP(III)PhosphorodiamiditeP(V)Phosphorodiamidate (e.g. Morpholino, Cyclophosphamide)Three P-N bondP(V)Phosphoramide (e.g. Hexamethylphosphoramide, Metepa)With P-C and P-N bonds One P-C bond and one P-N bondP(V)PhosphonamidateOne P-C bond and two P-N bondP(V)PhosphonamideTwo P-C bond and one P-N bondP(V)PhosphinamideWith P-S bonds One P-S bondP(III)PhosphorothioiteP(V)PhosphorothioateTwo P-S bondP(III)PhosphorodithioiteP(V)PhosphorodithioateCategories:- Phosphorothioates
- Anions
Wikimedia Foundation. 2010.