- Peptidylglycine alpha-amidating monooxygenase
-
Peptidyl-glycine alpha-amidating monooxygenase is an enzyme that in humans is encoded by the PAM gene.[1][2]
This gene encodes a multifunctional protein. It has two enzymatically active domains with catalytic activities - peptidylglycine alpha-hydroxylating monooxygenase (PHM) and peptidyl-alpha-hydroxyglycine alpha-amidating lyase (PAL). These catalytic domains work sequentially to catalyze neuroendocrine peptides to active alpha-amidated products. Multiple alternatively spliced transcript variants encoding different isoforms have been described for this gene but some of their full length sequences are not yet known.[2]
Interactions
Peptidylglycine alpha-amidating monooxygenase has been shown to interact with TSC2.[3]
References
- ^ Glauder J, Ragg H, Rauch J, Engels JW (Jul 1990). "Human peptidylglycine alpha-amidating monooxygenase: cDNA, cloning and functional expression of a truncated form in COS cells". Biochem Biophys Res Commun 169 (2): 551–8. doi:10.1016/0006-291X(90)90366-U. PMID 2357221.
- ^ a b "Entrez Gene: PAM peptidylglycine alpha-amidating monooxygenase". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5066.
- ^ Murthy, Vanishree; Han Sangyeul, Beauchamp Roberta L, Smith Nicole, Haddad Luciana A, Ito Naoto, Ramesh Vijaya (Jan. 2004). "Pam and its ortholog highwire interact with and may negatively regulate the TSC1.TSC2 complex". J. Biol. Chem. (United States) 279 (2): 1351–8. doi:10.1074/jbc.M310208200. ISSN 0021-9258. PMID 14559897.
Further reading
- Pittner RA, Albrandt K, Beaumont K, et al. (1994). "Molecular physiology of amylin.". J. Cell. Biochem.. 55 Suppl (S1994A): 19–28. doi:10.1002/jcb.240550004. PMID 7929615.
- Eipper BA, Milgram SL, Husten EJ, et al. (1993). "Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains.". Protein Sci. 2 (4): 489–97. doi:10.1002/pro.5560020401. PMC 2142366. PMID 8518727. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2142366.
- Ouafik LH, Stoffers DA, Campbell TA, et al. (1992). "The multifunctional peptidylglycine alpha-amidating monooxygenase gene: exon/intron organization of catalytic, processing, and routing domains.". Mol. Endocrinol. 6 (10): 1571–84. doi:10.1210/me.6.10.1571. PMID 1448112.
- Maltese JY, Eipper BA (1993). "Developmental expression of peptidylglycine alpha-amidating monooxygenase (PAM) in primary cultures of neonatal rat cardiocytes: a model for studying regulation of PAM expression in the rat heart.". Mol. Endocrinol. 6 (12): 1998–2008. doi:10.1210/me.6.12.1998. PMID 1491686.
- Braas KM, Harakall SA, Ouafik L, et al. (1992). "Expression of peptidylglycine alpha-amidating monooxygenase: an in situ hybridization and immunocytochemical study.". Endocrinology 130 (5): 2778–88. doi:10.1210/en.130.5.2778. PMID 1572293.
- Roberts AN, Leighton B, Todd JA, et al. (1990). "Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus.". Proc. Natl. Acad. Sci. U.S.A. 86 (24): 9662–6. doi:10.1073/pnas.86.24.9662. PMC 298561. PMID 2690069. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=298561.
- Vos MD, Jones JE, Treston AM (1995). "Human peptidylglycine alpha-amidating monooxygenase transcripts derived by alternative mRNA splicing of an unreported exon.". Gene 163 (2): 307–11. doi:10.1016/0378-1119(95)00364-C. PMID 7590286.
- Tsukamoto T, Noguchi M, Kayama H, et al. (1995). "Increased peptidylglycine alpha-amidating monooxygenase activity in cerebrospinal fluid of patients with multiple sclerosis.". Intern. Med. 34 (4): 229–32. doi:10.2169/internalmedicine.34.229. PMID 7606087.
- Yun HY, Johnson RC, Mains RE, Eipper BA (1993). "Topological switching of the COOH-terminal domain of peptidylglycine alpha-amidating monooxygenase by alternative RNA splicing.". Arch. Biochem. Biophys. 301 (1): 77–84. doi:10.1006/abbi.1993.1117. PMID 7680192.
- Mains RE, Milgram SL, Keutmann HT, Eipper BA (1995). "The NH2-terminal proregion of peptidylglycine alpha-amidating monooxygenase facilitates the secretion of soluble proteins.". Mol. Endocrinol. 9 (1): 3–13. doi:10.1210/me.9.1.3. PMID 7760848.
- Tateishi K, Arakawa F, Misumi Y, et al. (1995). "Isolation and functional expression of human pancreatic peptidylglycine alpha-amidating monooxygenase.". Biochem. Biophys. Res. Commun. 205 (1): 282–90. doi:10.1006/bbrc.1994.2662. PMID 7999037.
- Martínez A, Montuenga LM, Springall DR, et al. (1993). "Immunocytochemical localization of peptidylglycine alpha-amidating monooxygenase enzymes (PAM) in human endocrine pancreas.". J. Histochem. Cytochem. 41 (3): 375–80. PMID 8094086.
- Kapuscinski M, Green M, Sinha SN, et al. (1993). "Peptide alpha-amidation activity in human plasma: relationship to gastrin processing.". Clin. Endocrinol. (Oxf) 39 (1): 51–8. doi:10.1111/j.1365-2265.1993.tb01750.x. PMID 8102327.
- Yun HY, Keutmann HT, Eipper BA (1994). "Alternative splicing governs sulfation of tyrosine or oligosaccharide on peptidylglycine alpha-amidating monooxygenase.". J. Biol. Chem. 269 (14): 10946–55. PMID 8144680.
- Ouafik LH, Mattei MG, Giraud P, et al. (1994). "Localization of the gene encoding peptidylglycine alpha-amidating monooxygenase (PAM) to human chromosome 5q14-5q21.". Genomics 18 (2): 319–21. doi:10.1006/geno.1993.1471. PMID 8288234.
- Husten EJ, Tausk FA, Keutmann HT, Eipper BA (1993). "Use of endoproteases to identify catalytic domains, linker regions, and functional interactions in soluble peptidylglycine alpha-amidating monooxygenase.". J. Biol. Chem. 268 (13): 9709–17. PMID 8486658.
- Yun HY, Milgram SL, Keutmann HT, Eipper BA (1996). "Phosphorylation of the cytosolic domain of peptidylglycine alpha-amidating monooxygenase.". J. Biol. Chem. 270 (50): 30075–83. doi:10.1074/jbc.270.50.30075. PMID 8530412.
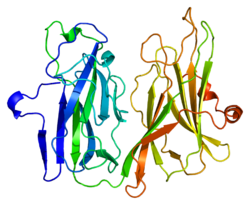
PDB gallery 1opm: OXIDIZED (CU2+) PEPTIDYLGLYCINE ALPHA-HYDROXYLATING MONOOXYGENASE (PHM) WITH BOUND SUBSTRATE1phm: PEPTIDYLGLYCINE ALPHA-HYDROXYLATING MONOOXYGENASE (PHM) FROM RAT1sdw: Reduced (Cu+) peptidylglycine alpha-hydroxylating monooxygenase with bound peptide and dioxygen1yi9: Crystal Structure Analysis of the oxidized form of the M314I mutant of Peptidylglycine alpha-Hydroxylating Monooxygenase1yip: Oxidized Peptidylglycine Alpha-Hydroxylating Monooxygenase (PHM) in a New Crystal Form1yjk: Reduced Peptidylglycine Alpha-Hydroxylating Monooxygenase (PHM) in a New Crystal Form1yjl: Reduced Peptidylglycine alpha-Hydroxylating Monooxygenase in a new crystal form3phm: REDUCED (CU+) PEPTIDYLGLYCINE ALPHA-HYDROXYLATING MONOOXYGENASE (PHM)Categories:- Human proteins
- Chromosome 5 gene stubs
Wikimedia Foundation. 2010.