- Defensin, alpha 1
-
Defensin, alpha 1 
PDB rendering based on 1dfn.Available structures PDB 2KHT, 2PM1, 3GNY, 3H6C, 3HJ2, 3HJD, 3LO1, 3LO2, 3LO4, 3LO6, 3LO9, 3LOE, 3LVX Identifiers Symbols DEFA1; DEF1; DEFA1B; DEFA2; HNP-1; HP-1; HP1; MGC138393; MRS External IDs OMIM: 125220 HomoloGene: 121760 GeneCards: DEFA1 Gene Gene Ontology Cellular component • extracellular region Biological process • xenobiotic metabolic process
• chemotaxis
• immune response
• response to virus
• defense response to bacterium
• defense response to fungusSources: Amigo / QuickGO RNA expression pattern 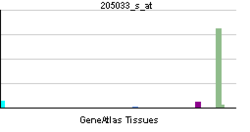
More reference expression data Orthologs Species Human Mouse Entrez 1667 n/a Ensembl ENSG00000185918 n/a UniProt P59665 n/a RefSeq (mRNA) NM_004084 n/a RefSeq (protein) NP_004075 n/a Location (UCSC) Chr 8:
6.86 – 6.86 Mbn/a PubMed search [1] n/a Defensin, alpha 1 also known as human alpha defensin 1, human neutrophil peptide 1 (HNP-1) or neutrophil defensin 1 is a human protein that is encoded by the DEFA1 gene.[1][2][3] Human alpha defensin 1 belongs to the alpha defensin family of antimicrobial peptides.
Function
Defensins are a family of microbicidal and cytotoxic peptides thought to be involved in host defense. They are abundant in the granules of neutrophils and also found in the epithelia of mucosal surfaces such as those of the intestine, respiratory tract, urinary tract, and vagina. Members of the defensin family are highly similar in protein sequence and distinguished by a conserved cysteine motif. Several alpha defensin genes are clustered on chromosome 8. The protein encoded by this gene, defensin, alpha 1, is found in the microbicidal granules of neutrophils and likely plays a role in phagocyte-mediated host defense. It differs from the defensins, alpha 2 and alpha 3 by only one amino acid.[3]
References
- ^ Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM (Mar 1997). "Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes". J Neurosci 17 (5): 1539–47. PMID 9030614.
- ^ Aldred PM, Hollox EJ, Armour JA (Jul 2005). "Copy number polymorphism and expression level variation of the human alpha-defensin genes DEFA1 and DEFA3". Hum Mol Genet 14 (14): 2045–52. doi:10.1093/hmg/ddi209. PMID 15944200.
- ^ a b "Entrez Gene: DEFA1 defensin, alpha 1". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1667.
Further reading
- Lehrer RI, Lichtenstein AK, Ganz T (1993). "Defensins: antimicrobial and cytotoxic peptides of mammalian cells.". Annu. Rev. Immunol. 11: 105–28. doi:10.1146/annurev.iy.11.040193.000541. PMID 8476558.
- Corda D, Di Girolamo M (2003). "Mono-ADP-ribosylation: a tool for modulating immune response and cell signaling.". Sci. STKE 2002 (163): PE53. doi:10.1126/stke.2002.163.pe53. PMID 12488509.
- Valore EV, Ganz T (1992). "Posttranslational processing of defensins in immature human myeloid cells.". Blood 79 (6): 1538–44. PMID 1339298.
- Zhang XL, Selsted ME, Pardi A (1992). "NMR studies of defensin antimicrobial peptides. 1. Resonance assignment and secondary structure determination of rabbit NP-2 and human HNP-1.". Biochemistry 31 (46): 11348–56. doi:10.1021/bi00161a012. PMID 1445872.
- Pardi A, Zhang XL, Selsted ME, et al. (1992). "NMR studies of defensin antimicrobial peptides. 2. Three-dimensional structures of rabbit NP-2 and human HNP-1.". Biochemistry 31 (46): 11357–64. doi:10.1021/bi00161a013. PMID 1445873.
- Hill CP, Yee J, Selsted ME, Eisenberg D (1991). "Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization.". Science 251 (5000): 1481–5. doi:10.1126/science.2006422. PMID 2006422.
- Bateman A, Singh A, Shustik C, et al. (1991). "The isolation and identification of multiple forms of the neutrophil granule peptides from human leukemic cells.". J. Biol. Chem. 266 (12): 7524–30. PMID 2019582.
- Wagner MJ, Ge Y, Siciliano M, Wells DE (1991). "A hybrid cell mapping panel for regional localization of probes to human chromosome 8.". Genomics 10 (1): 114–25. doi:10.1016/0888-7543(91)90491-V. PMID 2045096.
- Sparkes RS, Kronenberg M, Heinzmann C, et al. (1989). "Assignment of defensin gene(s) to human chromosome 8p23.". Genomics 5 (2): 240–4. doi:10.1016/0888-7543(89)90052-9. PMID 2793180.
- Selsted ME, Harwig SS (1989). "Determination of the disulfide array in the human defensin HNP-2. A covalently cyclized peptide.". J. Biol. Chem. 264 (7): 4003–7. PMID 2917986.
- Wiedemann LM, Francis GE, Lamb RF, et al. (1989). "Differentiation stage-specific expression of a gene during granulopoiesis.". Leukemia 3 (3): 227–34. PMID 2918759.
- Ganz T, Selsted ME, Szklarek D, et al. (1985). "Defensins. Natural peptide antibiotics of human neutrophils.". J. Clin. Invest. 76 (4): 1427–35. doi:10.1172/JCI112120. PMC 424093. PMID 2997278. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=424093.
- Daher KA, Lehrer RI, Ganz T, Kronenberg M (1988). "Isolation and characterization of human defensin cDNA clones.". Proc. Natl. Acad. Sci. U.S.A. 85 (19): 7327–31. doi:10.1073/pnas.85.19.7327. PMC 282179. PMID 3174637. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=282179.
- Mars WM, van Tuinen P, Drabkin HA, et al. (1988). "A myeloid-related sequence that localizes to human chromosome 8q21.1-22.". Blood 71 (6): 1713–9. PMID 3370315.
- Selsted ME, Harwig SS, Ganz T, et al. (1985). "Primary structures of three human neutrophil defensins.". J. Clin. Invest. 76 (4): 1436–9. doi:10.1172/JCI112121. PMC 424095. PMID 4056036. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=424095.
- Panyutich AV, Hiemstra PS, van Wetering S, Ganz T (1995). "Human neutrophil defensin and serpins form complexes and inactivate each other.". Am. J. Respir. Cell Mol. Biol. 12 (3): 351–7. PMID 7873202.
- Date Y, Nakazato M, Shiomi K, et al. (1994). "Localization of human neutrophil peptide (HNP) and its messenger RNA in neutrophil series.". Ann. Hematol. 69 (2): 73–7. doi:10.1007/BF01698485. PMID 8080882.
- Linzmeier R, Michaelson D, Liu L, Ganz T (1993). "The structure of neutrophil defensin genes.". FEBS Lett. 326 (1–3): 299–300. doi:10.1016/0014-5793(93)81813-F. PMID 8325384.
- Linzmeier R, Michaelson D, Liu L, Ganz T (1993). "The structure of neutrophil defensin genes". FEBS Lett. 321 (2–3): 267–73. doi:10.1016/0014-5793(93)80122-B. PMID 8477861.
PDB gallery 1dfn: CRYSTAL STRUCTURE OF DEFENSIN HNP-3, AN AMPHIPHILIC DIMER: MECHANISMS OF MEMBRANE PERMEABILIZATION1zmh: Crystal structure of human neutrophil peptide 2, HNP-2 (variant Gly16-> D-Ala)1zmi: Crystal structure of human alpha_defensin-2 (variant GLY16->D-ALA), P 32 2 1 space group )1zmk: Crystal structure of human alpha-defensin-2 (variant Gly16-> D-ALA), P 42 21 2 space group2pm1: Derivative of human alpha-defensin 1 (HNP1)2pm4: Human alpha-defensin 1 (multiple Arg->Lys mutant)2pm5: Human alpha-defensin 1 derivative (HNP1)Categories:- Human proteins
- Chromosome 8 gene stubs
Wikimedia Foundation. 2010.