- Chromium nitrate
-
Chromium nitrate 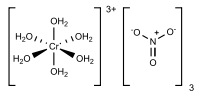 Chromium(III) nitrateOther namesNitric acid, chromium(3+) salt
Chromium(III) nitrateOther namesNitric acid, chromium(3+) saltIdentifiers CAS number 13548-38-4  , 7789-02-8 (nonahydrate)
, 7789-02-8 (nonahydrate) 
PubChem 24598 ChemSpider 15285818 
UNII C6H0RE016B 
UN number 2720 RTECS number GB6300000 Jmol-3D images Image 1 - [Cr+2].O=N([O-])=O.[O-]N(=O)=O.[O-]N(=O)=O
Properties Molecular formula Cr(NO3)3
[Cr(H2O)6](NO3)3•3H2O (nonahydrate)Molar mass 238.011 g/mol (anhydrous)
400.21 g/mol (nonahydrate)Appearance Blue-violet crystals (anhydrous)
Purple crystals (nonahydrate)Density 1.85 g/cm3 (nonahydrate) Melting point 60.06 °C, 333 K, 140 °F (nonahydrate)
Boiling point > 100 °C (212 °F) (decomp.)
Solubility in water 81 g/100 mL (20 °C) Hazards MSDS Oxford MSDS EU Index Not listed NFPA 704 Flash point Non flammable LD50 3250 mg/kg (rat, oral, nonahydrate)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chromium(III) nitrate describes several inorganic compounds consisting of chromium, nitrate and varying amounts of water. Most common is the dark violet hydrated solid, but an anhydrous green form is also known. These compounds are not important commercially but are commonly found in academic laboratories.
Contents
Structure
The relatively complicated formula - [Cr(H2O)6](NO3)3•3H2O - highlights the complicated structure of this material. The chromium centers are bound to six water ligands, and the remaining volume of the solid is occupied by three nitrate anions and three molecules of water of crystallization. Such complicated formulas typify hydrated metal salts.
Properties
The anhydrous salt forms green crystals and very soluble in water. At 100 °C it decomposes. The red-violet hydrate is highly soluble in water. Chromium nitrate is used in the production of alkali metal-free catalysts and in pickling.
Preparation
Chromium nitrate can be prepared by dissolving chromium oxide in nitric acid.[1]
References
- ^ Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
Chromium compounds Categories:- Chromium compounds
- Nitrates
- Oxidizing agents
Wikimedia Foundation. 2010.

