- Chromium(III) fluoride
-
Chromium(III) fluoride 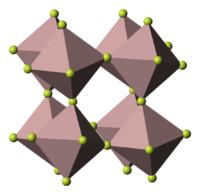
 Chromium(III) fluoride
Chromium(III) fluorideIdentifiers CAS number 7788-97-8, 16671-27-5 (trihydrate), 123333-98-2 (tetrahydrate) PubChem 10154021 ChemSpider 8329529 
RTECS number GB6125000 Jmol-3D images Image 1
Image 2- [Cr+2].[F-].[F-].[F-]
[Cr+3].[F-].[F-].[F-]
Properties Molecular formula CrF3 Molar mass 108.9913 g/mol (anhydrous), 163.037 g/mol (trihydrate), 181.05 g/mol (tetrahydrate) Appearance green crystalline solid Density 3.8 g/cm3 (anhydrous), 2.2 g/cm3 (trihydrate) Melting point 1100 °C (sublimes)
Solubility in water negligible (anhydrous), sparingly soluble (trihydrate) Solubility insoluble and alcohols
soluble in HF, HClStructure Crystal structure Rhombohedral, hR24 Space group R-3c, No. 167  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chromium(III) fluoride is the name for the inorganic compounds with the formula CrF3 as well as several related hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the coloured hydrates [Cr(H2O)6]F3 and [Cr(H2O)6]F3.3H2O are soluble in water. The trihydrate is green, and the hexahydrate is violet The anhydrous form sublimes at 1100-1200 °C. Like almost all compounds of chromium(III), these compounds feature octahedral Cr centres. In the anhydrous form, the six coordination sites are occupied by fluoride ligands that bridge to adjacent Cr centres. In the hydrates, some or all of the fluoride ligands are replaced by water.[1]
Production
Chromium(III) fluoride is produced from the reaction of chromium(III) oxide and hydrofluoric acid:[2]
- Cr2O3 + 6 HF + 9 H2O → 2 [Cr(H2O)6]F3
The anhydrous form is produced from hydrogen fluoride and chromic chloride:[3]
- CrCl3 + 3 HF → CrF3 + 3 HCl
Uses
Chromium(III) fluoride is not heavily used, but finds some applications as a mordant in textiles and as a corrosion inhibitor.
References
- ^ F.H. Herbstein, M. Kapon and G.M. Reisner, "Crystal structures of chromium(III) fluoride trihydrate. Structural chemistry of hydrated transition metal fluorides. Thermal decomposition of chromium(III) fluoride nonhydrate" Zeitschrift für Kristallographie 1985, volume 171, pp. 209
- ^ Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a07_067
- ^ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
Chromium compounds Categories:- Fluorides
- Chromium compounds
- Inorganic compound stubs
- [Cr+2].[F-].[F-].[F-]
Wikimedia Foundation. 2010.
