- Dehydroacetic acid
-
Dehydroacetic acid[1] 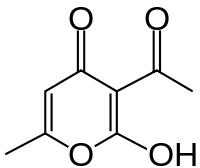 Other names
Other namesIdentifiers Abbreviations DHAA CAS number 520-45-6 
PubChem 10623 ChemSpider 10177 
UNII 2KAG279R6R 
EC number 208-293-9 MeSH dehydroacetic+acid Jmol-3D images Image 1 - CC(=O)C1=C(O)OC(C)=CC1=O
Properties Molecular formula C8H8O4 Molar mass 168.15 g mol−1 Exact mass 168.042258744 g mol-1 Appearance White crystals Melting point 109 °C, 382 K, 228 °F
Boiling point 270 °C, 543 K, 518 °F
Hazards EU Index 607-163-00-2 EU classification  Xn
XnR-phrases R22 S-phrases (S2)  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dehydroacetic acid is a pyrone derivative used mostly as a fungicide and bactericide. It is used to reduce pickle bloating as a preservative for squash and strawberries.[2]
Also used in antienzyme toothpastes.The sodium salt, sodium dehydroacetate, is often used in place of dehydroacetic acid because of its greater solubility in water.
Industrially, it is also used as a plasticizer in a variety of synthetic resins.[1]
References
- ^ a b Merck Index, 11th Edition, 2855
- ^ Handbook of Biocide and Preservative Use, Harold William Rossmoore, p. 341 ISBN 0751402125

This article about an alcohol is a stub. You can help Wikipedia by expanding it. This article about a heterocyclic compound is a stub. You can help Wikipedia by expanding it. This article about a ketone is a stub. You can help Wikipedia by expanding it.
