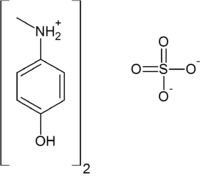- Metol
-
Metol 4-(methylamino)phenol sulfateOther namesN-methyl-p-aminophenol sulfate, p-(methylamino)phenol sulfate, monomethyl-p-aminophenol hemisulfate, Metol, Elon, Rhodol, Enel, Viterol, Scalol, Genol, Satrapol.Identifiers CAS number 55-55-0 
PubChem 5930 ChemSpider 5717 
UNII D3W0VWG12M 
KEGG C11206 
ChEBI CHEBI:55413 
Jmol-3D images Image 1 - O=S(=O)(O)O.Oc1ccc(NC)cc1.Oc1ccc(NC)cc1
Properties Molecular formula (C7H10NO)2SO4 Molar mass 344.38 g/mol Melting point 260 °C, 533 K, 500 °F
Hazards MSDS Oxford MSDS EU classification  T
T
 N
N (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Metol is the chemical compound with the name monomethyl-p-aminophenol hemisulfate. It is a developing agent used in black & white photographic developers. In its pure form, it is a solid rather light-sensitive chemical which is the half sulfate (hemisulfate) salt of N-methyl-p-aminophenol.
Contents
Synthesis
Metol has been prepared by the reaction of hydroquinone with methylamine, followed by neutralization with sulfuric acid.[1]
Application
Metol is an excellent developing agent for most continuous tone developer applications, and it has been widely used in published developer formulas as well as commercial products. However, it is difficult to produce highly concentrated developer solutions using Metol, and therefore most Metol developers are supplied in dry chemical mix. A developer containing both Metol and hydroquinone is called an MQ developer. This combination of agents provide greater developer activity since the rate of development by both agents together is greater than the sum of rates of developments by each agent used alone (superadditivity). This combination is very versatile; by varying the quantities of Metol, hydroquinone, restrainer, and adjusting the pH, the entire range of continuous tone developers can be made. Therefore, Metol replaced most other developing agents except for hydroquinone, Phenidone (which is more recent than Metol), and derivatives of Phenidone. Notable formulas include Eastman Kodak D-76 film developer, D-72 print developer, and D-96 motion picture negative developer.
History
Alfred Bogisch discovered in 1891 that methylated p-aminophenol has more vigorous developing action than p-aminophenol, and Julius Hauff introduced this compound as a developing agent. (Hauff operated a chemical business for which Bogisch worked.) Exact description of Bogisch and Hauff's early Metol is indefinitive, but it is most likely methylated at the ortho position of the phenol group (p-amino-o-methylphenol), rather than at the amino group. Some time after, Metol became to mean N-methylated variety and o-methylated variety had fallen out of use. Aktien-Gesellschaft fuer Anilinfabrikation (AGFA) sold this compound under trade name Metol and it became by far the most common name, followed by Eastman Kodak's trade name Elon.
Because it has been in use for this purpose for over 100 years and often by amateur photographers, there is a wide body of evidence about the health problems that contact with Metol can cause. These are principally local dermatitis of the hands and fore-arms as well as some evidence of sensitization dermatitis in which subsequent exposure triggers of a chronic condition that is resistant to medication. The use of Metol in highly caustic solutions and the presence of other materials in darkrooms that have been implicated in dermatitis such as Cr(VI) salts may exacerbate some health impacts.
References
- ^ Harger, Rolla N. (1919). "Preparation of Metol". J. Am. Chem. Soc. 41: 270. doi:10.1021/ja01459a014.
Categories:- Photographic chemicals
- Phenols
Wikimedia Foundation. 2010.