- Copper benzoate
-
Copper benzoate 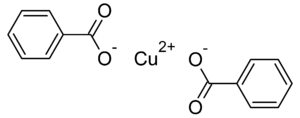

 copper dibenzoateOther namescupric benzoate
copper dibenzoateOther namescupric benzoateIdentifiers CAS number 533-01-7 PubChem 164650 ChemSpider 144339 
Jmol-3D images Image 1 - [Cu+2].[O-]C(=O)c1ccccc1.[O-]C(=O)c1ccccc1
Properties Molecular formula C14H10CuO4 Molar mass 305.7728 g/mol Appearance blue solid Density 1.197g/cm3 Boiling point 249.3°C @760mmHg
Hazards Flash point 111.4°C Related compounds Other cations sodium benzoate  benzoate (verify) (what is:
benzoate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Copper benzoate is the chemical compound with the formula Cu(C6H5CO2)2. This coordination complex is derived from the cupric ion and the conjugate base of benzoic acid. Because copper emits blue when heated in a flame, this salt has found some use as a source of blue light in fireworks.[1]
Preparation and structure
As for copper(II) acetate,[2] the benzoate adopts a "Chinese lantern" structure, wherein a pair of copper centers are linked by four bridging carboxylate ligands. Typically one site on each copper center is occupied by water, which can be replaced by other ligands.
References
- ^ "Wouter's Practical Pyrotechnics page". http://www.wfvisser.dds.nl/EN/cheminfo_EN.html#copperbenzoate.
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
Categories:- Copper compounds
- Benzoates
Wikimedia Foundation. 2010.
