- Glycidol
-
Glycidol[1] 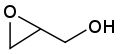 OxiranylmethanolOther namesGlycidol
OxiranylmethanolOther namesGlycidol
2,3-Epoxy-1-propanol
3-Hydroxypropylene oxideIdentifiers CAS number 556-52-5 
PubChem 11164 ChemSpider 10691 
KEGG C10920 
ChEBI CHEBI:30966 
Jmol-3D images Image 1 - OCC1OC1
Properties Molecular formula C3H6O2 Molar mass 74.08 g mol−1 Density 1.1143 g/cm³ Melting point -54 °C
Boiling point 61-62 °C, 151 °F
Hazards MSDS External MSDS NFPA 704  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Glycidol is an organic compound that contains both epoxide and alcohol functional groups. Being bifunctional, it has a variety of industrial uses. The compound is a slightly viscous liquid that is slightly unstable and is not often encountered in pure form.
Contents
Synthesis and applications
Glycidol is prepared by the epoxidation of allyl alcohol.[2]
Glycidol is used as a stabilizer for natural oils and vinyl polymers and as a demulsifier. It is used as a chemical intermediate in the synthesis of glycerol, glycidyl ethers, esters and amines. It is used in surface coatings, chemical synthesis, pharmaceuticals, sanitary chemicals and sterilizing milk of magnesia, and as a gelation agent in solid propellants.[3]
Safety
Glycidol is an irritant of the skin, eyes, mucous membranes, and upper respiratory tract. Exposure to glycidol may also cause central nervous system depression, followed by central nervous system stimulation.[4] It is listed as IARC group2 carcinogen.
See also
References
- ^ Merck Index, 11th Edition, 4385.
- ^ Guenter Sienel, Robert Rieth, Kenneth T. Rowbottom "Epoxides" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a09_531
- ^ Glycidol at chemicalland21.com
- ^ OSHA guidelines for glycidol
Categories:- Epoxides
- Alcohols
- IARC Group 2A carcinogens
Wikimedia Foundation. 2010.

